Regeneration of Damaged Tissue
a tissue and repair technology, applied in the field of wound healing, can solve the problems of fibrosis and scar formation, other tissues are not capable of regeneration, and re-epithelialization must occur, so as to reduce scar tissue and minimize scar tissue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Dermal Regeneration Full Thickness Surgical Wounds
[0090]The current study assessed the impact of recombinant human tropoelastin (rH TE) on dermal regeneration of full thickness surgical wounds in a pig model following the application of Integra Dermal Template with and without rH TE. Analysis of the regenerated dermis at two weeks revealed that the presence of rH TE in Integra Dermal Template led to an improved wound repair process. The improvement was marked by increased numbers of fibroblast, elevated collagen deposition, increased vascularization of the regenerated dermal tissue, and an increased level of detected elastin fibers in the regenerated dermis. These events were accompanied by increased keratinocyte proliferation resulting in improved epithelialization of the wound due to the presence of rH TE.
[0091]Materials and Methods
[0092]Test Items
[0093]Three products were assessed in the current study:[0094]Control: Integra Dermal Template[0095]Test A: Integra Dermal Template inc...
example 2
Use of Electrospun, Co-Precipitate and Gel Based Formulations
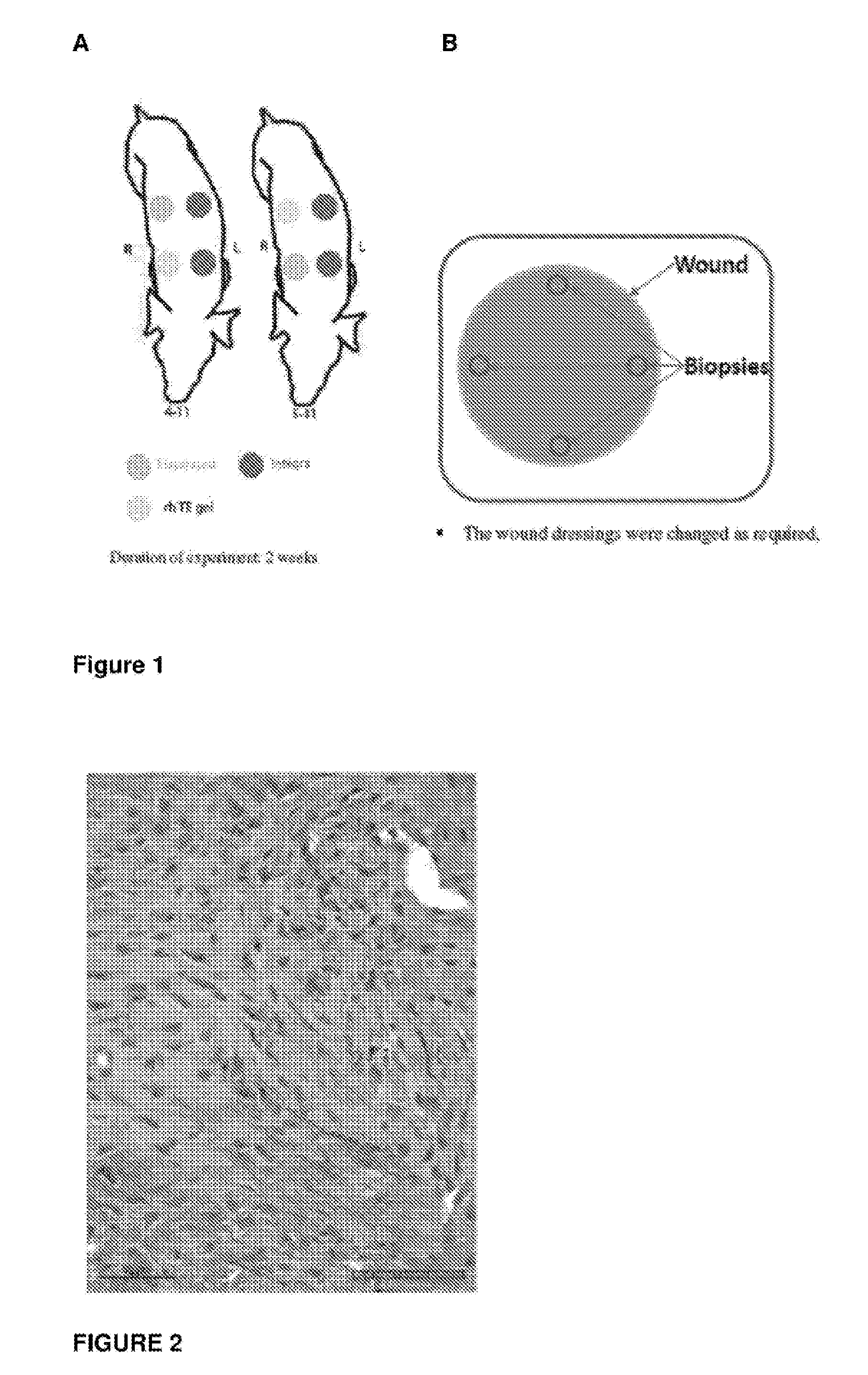
[0123]Pigs were utilized in the current study, each with four, circular, 5 cm diameter, wound sites, two on each side of the animal. For each pig, two wounds from one side were covered with a commercially available skin template product, and two wounds from the other side were treated with either test item A, B or C.[0124]Day 0[0125]Four full thickness excisional circular wounds with 5 cm in diameter were created on the upper backs of each pig as noted in the diagram above.[0126]Each wound was treated with either the control skin template or Test Item A, B or C.[0127]Day 7 (week 1)[0128]Dressing changes for all wounds.[0129]Day 14 (week 2)[0130]4 mm biopsies were taken from each wound site a few mm away from the edge of the wounds as depicted in FIG. 1B.
[0131]Wound Analysis
[0132]Sampling at the wound site was first undertaken two weeks after surgery and treatment. Biopsy of the wound site was conducted as described above. ...
example 3
Assessment of Epithelial Regeneration in a Porcine Needling Skin Model
[0139]Pigs were each treated with up to ten 2 cm×2 cm sites across the dorsum. Each site received one of three treatment methods:[0140]1) Site A: Received puncture wounds using a micro-coring needle approximately every 2 mm apart across the area of skin to be treated. Following the needle treatment a gel containing 1% to 5% w / v tropoelastin protein was applied topically to the treated area and held in place by a Tegaderm dressing to enable the gel to be retained and pass into the puncture sites.[0141]2) Site B: Received puncture wounds using a hypodermic needle with each puncture including the injection of 0.05 to 0.5 ml of a 1 to 5% w / v tropoelastin gel into the upper dermis of the skin tissue. The puncture / injections were applied approximately 2 mm apart across the area of skin to be treated followed by wound dressing.[0142]3) Site C: Received the implantation of 0.5 to 2 ml of a 1 to 5% w / v tropoelastin gel in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com