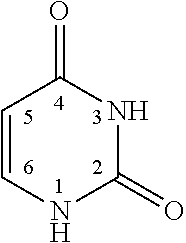
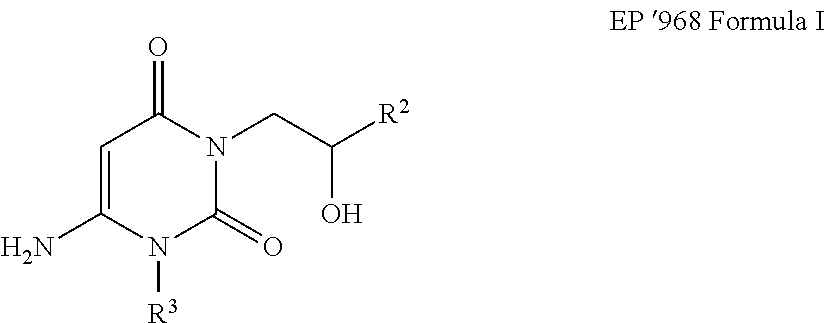
Novel coupled uracil compound for vinyl chloride polymer resins
a technology of vinyl chloride and compound, applied in the field of new coupled 6amino uracil derivative, can solve the problems of halogen containing polymers degrading or deteriorating when processed, halide acid generated by the polymer attacking the components of processing equipment, and halogen containing polymers tend to degrade or deteriorate when processed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0093]Sample 1—A commercially available compound of 6-amino-1,3-dimethyluracil, available from Sigma-Aldrich
[0094]Sample 2—Comparative dimer compound from Example 1 of U.S. Pat. No. 6,156,830, having a connection through a ring carbon
[0095]Sample 3—Comparative mono-functional alkylated Uracil derivative compound
[0096]Sample 4—Representative di-functional compound
examples 1 to 7
[0097]The samples were each compounded into a CPVC composition in an amount to achieve an equivalent or increased level of nitrogen compared to a 0.25 phr treat of sample 6 at control amount of Nitrogen (i.e., 1×N). The recipes for each example are shown in Table 1 below.
TABLE 1Ingredient13234567CPVC1 100 phr 100 phr 100 phr 100 phr 100 phr 100 phr 100 phrSample 1 0.25 phr(1.26xN)Sample 2 0.25 phr(1.22xN)Sample 3 0.41 phr 0.62 phr 0.99 phr(1xN)(1.5xN)(2.25xN)Sample 4 0.25 phr 0.4 phr(1xN)(1.5xN)Additive15.515 phr16.015 phr16.015 phr16.015 phr16.015 phr16.015 phr16.015 phrPackage2166.25 Cl % 0.92 inherent viscosity.27 phr impact modifier, 4 phr coated titanium dioxide, 1.5 phr type A zeolite, 1.0 phr disodium sebacate, 2.25 phr lubricant, 0.25 phr antioxidant, 0.015 phr yellow dye.3example 1 has 2.0 phr type A zeolite and no disodium sebacate.
[0098]Color chips of each example compound were prepared using a Brabender DTS mixing unit having a counter rotating batch mixing bowl....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com