Neuroactive steroids, compositions, and uses thereof
a technology applied in the field of neuroactive steroids and compositions, to achieve the effect of reducing depression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Human Phase 1 Study of Allopregnanolone
[0358]A Double-Blind, Placebo-Controlled, Two-Period Crossover, Proof-of-Concept Study Evaluating the Safety, Tolerability, Pharmacokinetics, Efficacy of Allopregnanolone Injection in the Treatment of Patients with Essential Tremor
Study Design
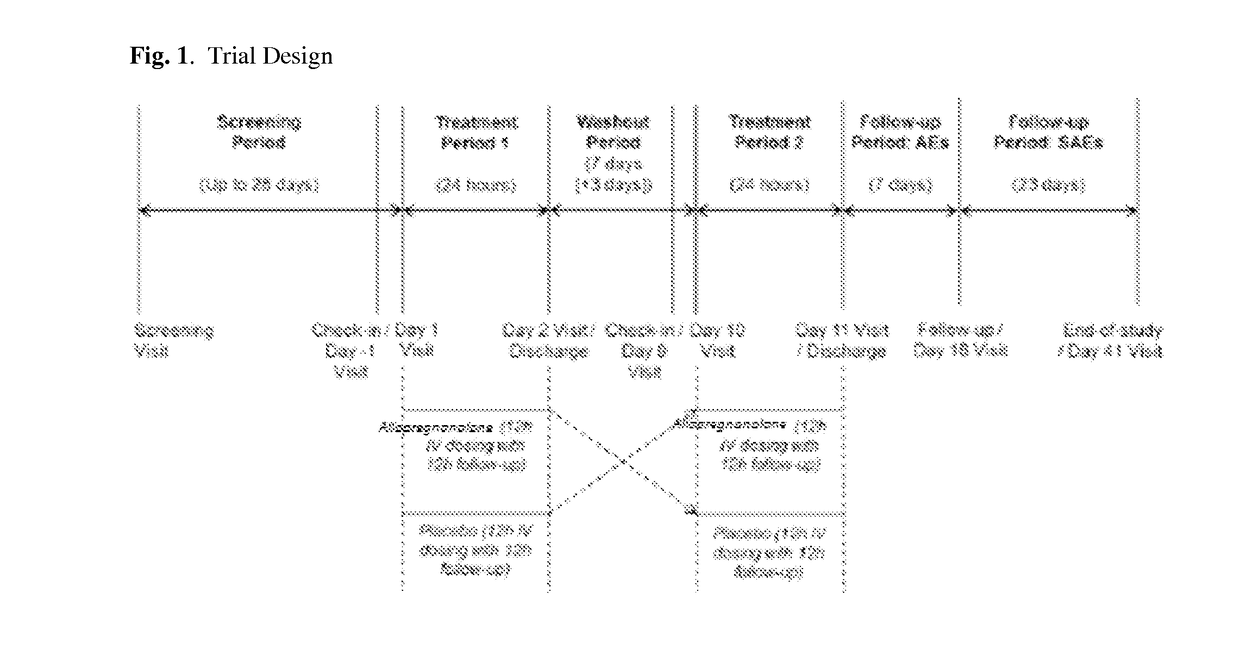
[0359]A double-blind, proof-of-concept study designed to evaluate the safety, tolerability, PK, and efficacy of allopregnanolone injection in male or female patients with essential tremor in the upper limb. Each patient's involvement is up to 72 days, including up to a 28-day Screening Period, 12-hour Treatment Period 1 with 12-hour follow-up, 7-day (+ / −3 days) Washout Period, 12-hour Treatment Period 2 with 12-hour follow-up, a 7-day Follow-up Visit (Day 18 + / −1 day) plus an additional 23 days of SAE follow-up (with an End-of-Study visit via call on day 41). A representation of the Trial Design and activities is shown on FIG. 1.
[0360]Screening Period: As depicted in FIG. 1, the Screening Period begins beg...
example 2
Human Phase 1 Study of Allopregnanolone
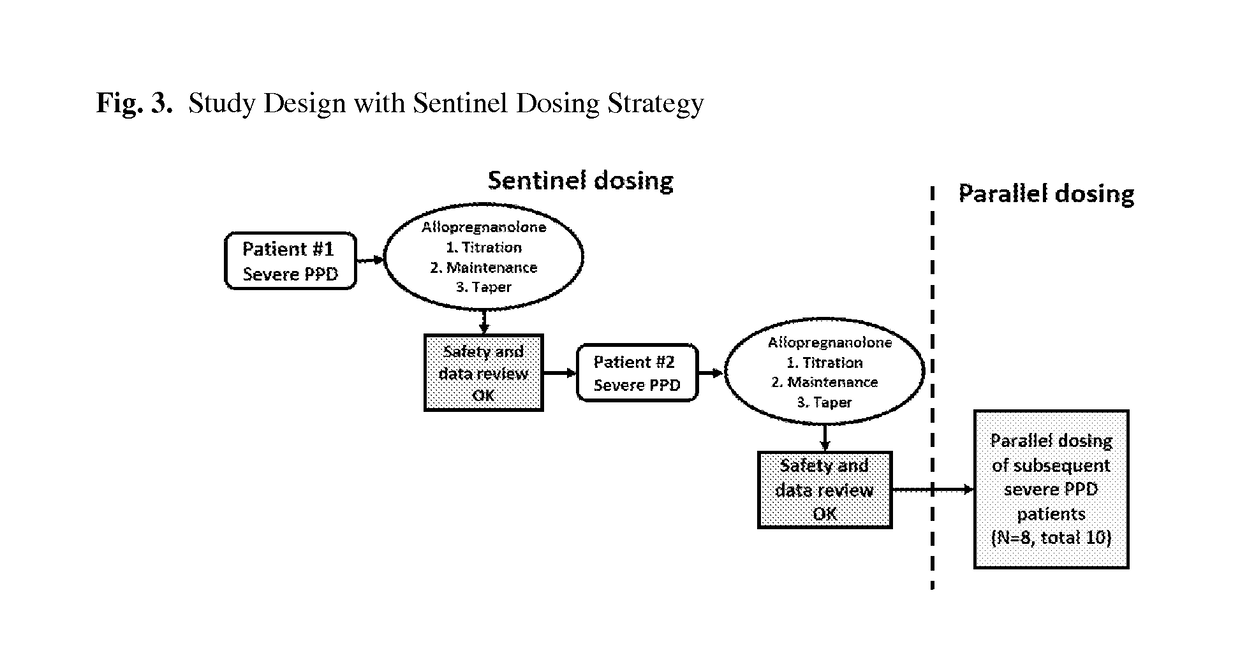
[0418]An Open-Label Proof-of-Concept Study Evaluating the Safety, Tolerability, Pharmacokinetics, and Efficacy of Allopregnanolone Injection in the Treatment of Adult Female Patients with Postpartum Depression
Study Design
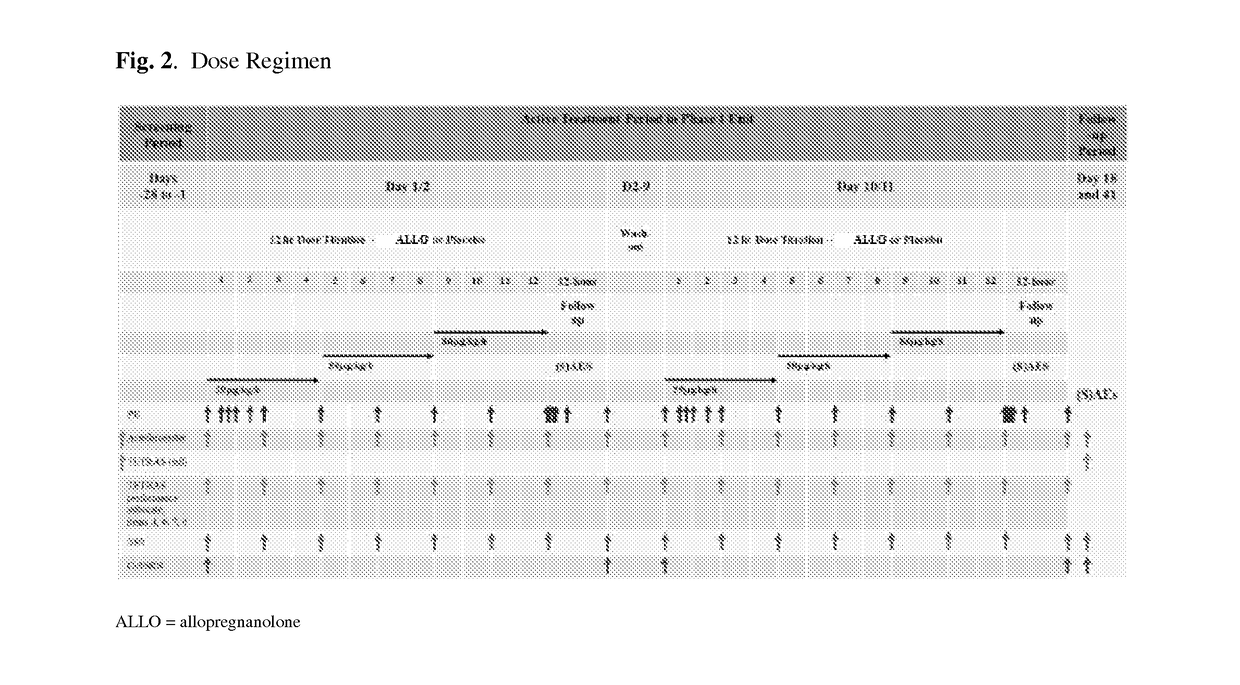
[0419]A double-blind, placebo-controlled study designed to evaluate the safety, tolerability, PK, and efficacy of allopregnanolone injection in adult female patients diagnosed (by Structured Clinical Interview for DSM-V Axis I Disorders [SCID-I]) with severe PPD. Each patient's involvement is up to 37 days, including up to a 3-day screening period, 4-day (84-hour) active treatment period, and a 7-day AE follow-up period plus an additional 23 days of SAE follow-up (with a telephone call at day 11 and day 34).
[0420]Screening Period: The Screening Period begins with the signature of the informed consent form (ICF) in the Perinatal Psychiatry Inpatient Unit (PPIU) at the Screening Visit, which can occur on any one calendar day of t...
example 3
A Double-Blind, Placebo-Controlled Study Evaluating the Efficacy, Safety, and Pharmacokinetics of Allopregnanolone Injection in the Treatment of Depression
Primary Objective:
[0484]To compare the effects of Allopregnanolone Injection and placebo infused intravenously for 48 hours on subject symptom response as measured by the Hamilton Rating Scale for Depression, 17-item (HAM-D-17)
Secondary Objectives:
[0485]To compare the effects of Allopregnanolone Injection and placebo on:[0486]Clinician evaluation as measured by the Clinical Global Impression-Improvement Scale (CGI-I)[0487]Sedation using the Stanford Sleepiness Scale (SSS)[0488]Safety and tolerability, assessed using adverse event reporting, vital sign measurement, laboratory data, ECG parameters, and suicidal ideation using the Columbia-Suicide Severity Rating Scale (C-SSRS)[0489]Depressive symptom severity, reproductive mood disorders, and sleepiness as measured by the following clinician- and subject-rated outcome measures: Edin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com