Composition of ginsenosides Rg3 and Rg5 and medicine use such as anti-tumor use of composition
A technology of ginsenoside and composition, which is applied in the field of composition preparation, can solve the problems of unidentified content, etc., and achieve the effects of improving immunity, antidepressant effect, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
[0055] The preparation of the composition of embodiment 1-9 ginsenoside Rg3 and ginsenoside Rg5
[0056] The specific process route is: decoct three times with water, combine the water and liquid, combine and concentrate to the crude drug: the water ratio is 1:5, keep the temperature at 60-80°C, add ZTC1+1-II clarifier (available from Wuhan Zhengtiancheng Biotechnology Co., Ltd.) to a final concentration of 500ppm, stir evenly, stand still, filter, dry the filtrate, add ethanol solution to dissolve at 60°C, cool down after filtering, precipitate in an ice-water bath, collect the precipitate, and dry it. The content of each component was detected by high performance liquid chromatography.
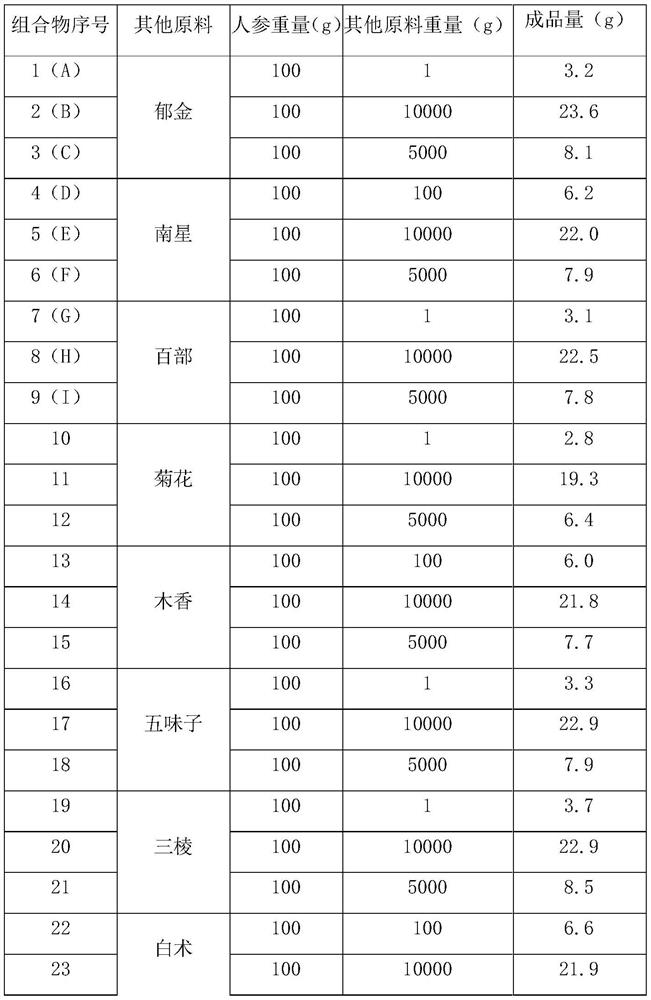
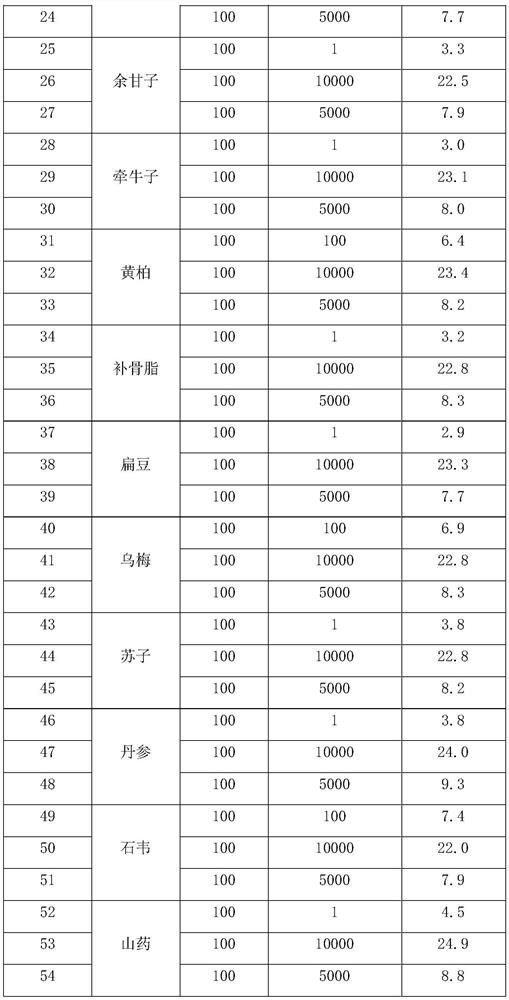
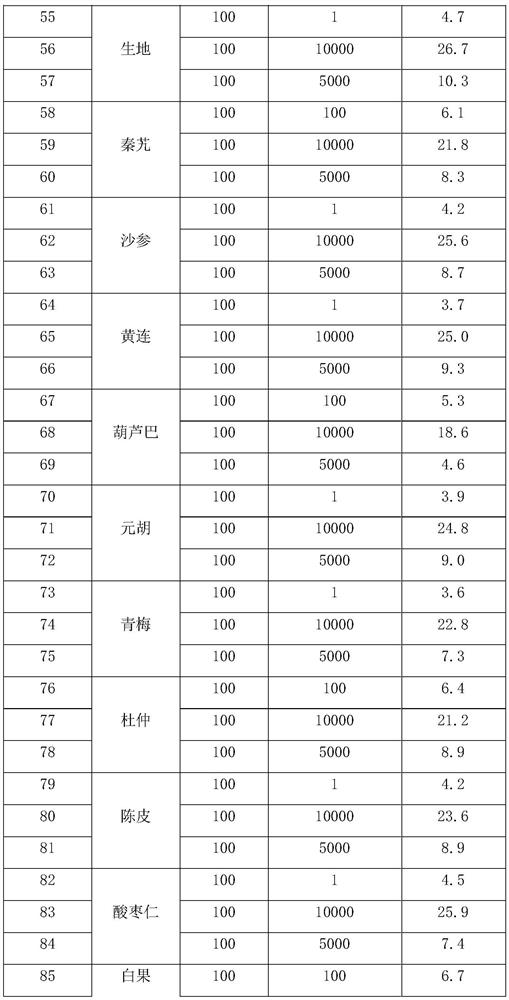
[0057] Ginseng and various crude drugs were weighed according to the weights in Table 1, and mixed and extracted with the parameters in Table 2 according to the above process route, and prepared. The test data of the finished product is shown in Table 3.
[0058] The raw material ratio of ...
Embodiment 181-198
[0081] Example 181-198 Preparation of ginsenoside Rg3 and ginsenoside Rg5 composition and cyclodextrin composition
[0082] Take the ginsenoside Rg3 and ginsenoside Rg5 compositions prepared in Example 1-198, and prepare the composition according to the following method with the weight ratio described in Table 4: (1) directly add to the cyclodextrin solution, or (2) directly Add to the cyclodextrin solution and stir thoroughly for 1-24h, (3) directly add to the cyclodextrin solution and heat for 10-120 minutes, (4) directly add to the cyclodextrin solution and sonicate for 10-120 minutes, (5) Grinding directly with cyclodextrin powder for 10-120 minutes, (6) mixing ginsenoside Rg3 and ginsenoside Rg5 with the cyclodextrin powder evenly, and sieving; (7) directly adding cyclodextrin to derivatize solution, or (8) directly added to the solution of cyclodextrin derivatives and fully stirred for 1-24h, (9) directly added to the solution of cyclodextrin derivatives and heated for 1...
Embodiment 199
[0086] The preparation of embodiment 199 ginsenoside Rg3 and ginsenoside Rg5 composition tablet
[0087] Prepare the tablet of ginsenoside Rg3 and ginsenoside Rg5 composition according to the following ratio:
[0088]
[0089] According to the above ratio, take the ginsenoside Rg3 and ginsenoside Rg5 compositions prepared by the process route in Example 1-198 and mix them evenly with starch, make granules, add talcum powder and magnesium stearate, mix evenly, and press into 10,000 tablets .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com