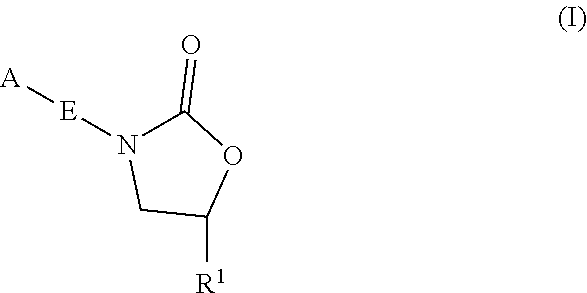
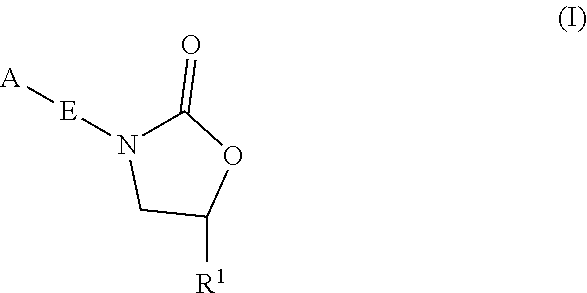
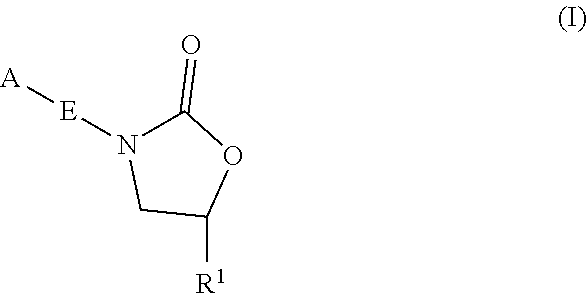
Oxazolidinone compounds and methods of use thereof as antibacterial agents
a technology of oxazolidinone and compounds, which is applied in the field of oxazolidinone compounds, can solve the problems of poor prognosis of infected aids patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of (S)-5-(aminomethyl)-3-(2-fluoro-4′-(methylthio)-[1,1′-biphenyl]-4-yl)oxazolidin-2-one
[0210]
Step A: Synthesis of (R)-3-(4-bromo-3-fluorophenyl)-5-(hydroxymethyl)oxazolidin-2-one
[0211]To a solution of benzyl (4-bromo-3-fluorophenyl)carbamate (50.0 g, 154 mmol) in THF (600 mL) was added lithium tert-butoxide (37.0 g, 463 mmol) at 0° C. The mixture was stirred at 20° C. for 2 hours, and then (R)-oxiran-2-ylmethyl butyrate (44.5 g, 309 mmol) at 20° C. was added. The resulting mixture was stirred at 20° C. for 16 hours, and then concentrated HCl was added to adjust the pH to 7. The organic solvent was removed under reduced pressure, and the residue was diluted with EtOAc (400 mL), washed with water (400 mL×2) and brine (500 mL), dried over Na2SO4, filtered and concentrated to give the crude product, which was purified by column chromatography (SiO2, PE:EtOAc=1:1) to give the product (R)-3-(4-bromo-3-fluorophenyl)-5-(hydroxymethyl)oxazolidin-2-one as a solid.
Step B: Synthesis ...
example 40
Synthesis of (S)—N-((3-(5-fluoro-6-(4-(methylthio)phenyl)pyridin-3-yl)-2-oxooxazolidin-5-yl)methyl)acetamide
[0217]
Step A: Synthesis of (S,E)-1-(benzylideneamino)-3-chloropropan-2-ol
[0218]To a solution of (S)-2-(chloromethyl)oxirane (5.0 g, 54.3 mmol) in EtOH (15 mL) was added ammonia hydrate (4.9 g, 140 mmol) and benzaldehyde (5.98 g, 56.4 mmol) and the resulting mixture was stirred at 40° C. for 16 hours. The reaction mixture was concentrated to give (S,E)-1-(benzylideneamino)-3-chloropropan-2-ol as a solid, which was used in the next step without purification.
Step B: Synthesis of (S)-1-amino-3-chloropropan-2-ol hydrochloride
[0219]To a solution of (S,E)-1-(benzylideneamino)-3-chloropropan-2-ol (6.2 g, 31.4 mmol) in EtOH (13 mL) and Toluene (12 mL) was added HCl (6.8 mL, 83 mmol) and the resulting mixture was stirred at 35° C. for 4 hours. The reaction mixture was concentrated to smaller volume, washed with water (20 mL), and the aqueous extract was lyophilized to give (S)-1-amino-3...
example 41
Synthesis of (S)—N-((3-(2-fluoro-4′-(methylthio)-[1,1′-biphenyl]-4-yl)-2-oxooxazolidin-5-yl)methyl)cyclopropanecarboxamide
[0223]
[0224]To a solution of cyclopropanecarboxylic acid (14.2 mg, 0.165 mmol) in DMF (2 mL) was added DIEA (53 mL, 0.301 mmol) and HATU (62.9 mg, 0.165 mmol). The mixture was stirred at 20° C. for 15 minutes and then (S)-5-(aminomethyl)-3-(2-fluoro-4′-(methylthio)-[1,1′-biphenyl]-4-yl)oxazolidin-2-one (50.0 mg, 0.150 mmol) was added. The mixture was stirred at 20° C. for 16 hours. The reaction mixture was filtered, and concentrated to give a residue which was purified by reverse phase prep-HPLC to give (S)—N-((3-(2-fluoro-4′-(methylthio)-[1,1′-biphenyl]-4-yl)-2-oxooxazolidin-5-yl)methyl)cyclopropanecarboxamide as a solid. LC / MS=401.1 [M+H+].
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| minimum inhibitory concentration | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com