Herpes simplex virus vaccine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0103]This example demonstrates the construction of an adenovirus comprising the inventive nucleic acid sequence encoding an HSV antigen.
[0104]A human serotype 28 adenoviral vector was prepared with a deletion in the E1 region, the replacement of the hexon region with the hexon region of a human serotype 45 adenovirus, and the insertion of the nucleic acid sequence comprising SEQ ID NO: 5 (Ad28UL19 H(Ad45)). Ad28UL19 H(Ad45) is replication-deficient due to the deletion of the essential function provided by E1. The CMV promoter and transgene expression cassette comprising SEQ ID NO: 5 were introduced in place of the E1 sequences. The expression cassette, located at the E1 region deletion junction, is right-to-left with respect to the viral genome.
[0105]Ad28UL19 H(Ad45) has an E1 region deletion of bases 462 through 3111 of the Ad28 genomic sequence. The deletion includes Ad28 E1A and part of the E1B early proteins, which renders the vector replication incompetent in noncomplementing ...
example 2
[0108]This example describes animal models for the evaluation of antiviral efficacy.
[0109]Mouse Model of Primary HSV-1 / HSV-2 Challenge:
[0110]The primary screening model provides a rapid initial evaluation of antiviral efficacy against HSV primary infection with both clinical and virological endpoints. This model utilizes intravaginal inoculation of female mice (25 g) with HSV-1 or HSV-2 to evaluate potential antiviral therapies as well as vaccine / adjuvant candidates. Animals are followed daily for signs and systems of herpes disease, and vaginal swabs are obtained to evaluate the effect of therapy on viral replication. Single or combined antiviral therapies can be administered by any suitable means, including, but not limited to, topically, orally or systemically, and can be given at varying intervals begun before or after viral challenge. Dose range studies also can be carried out.
[0111]Dose and route of administration are individualized for each experimental agent. However, for ea...
example 3
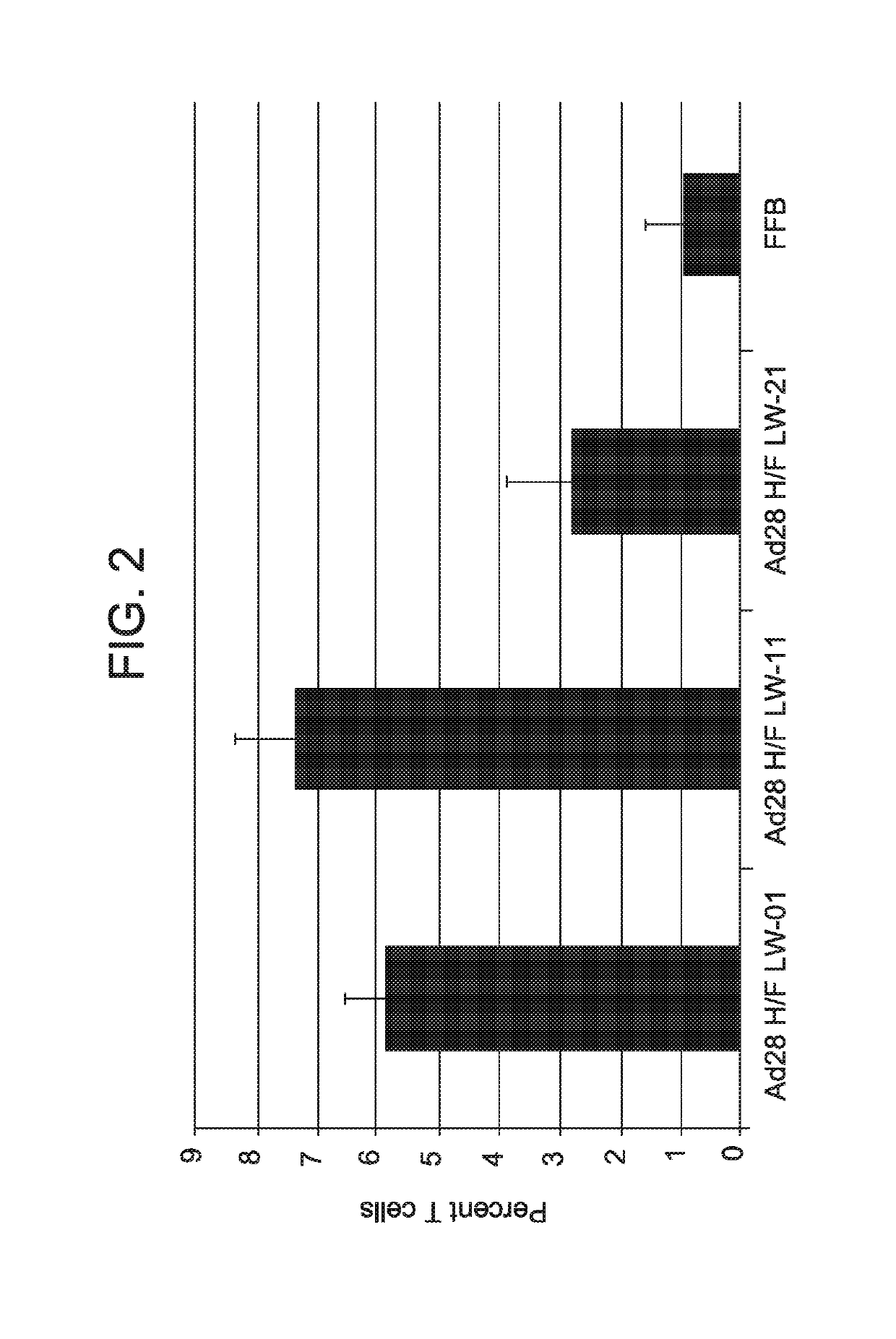
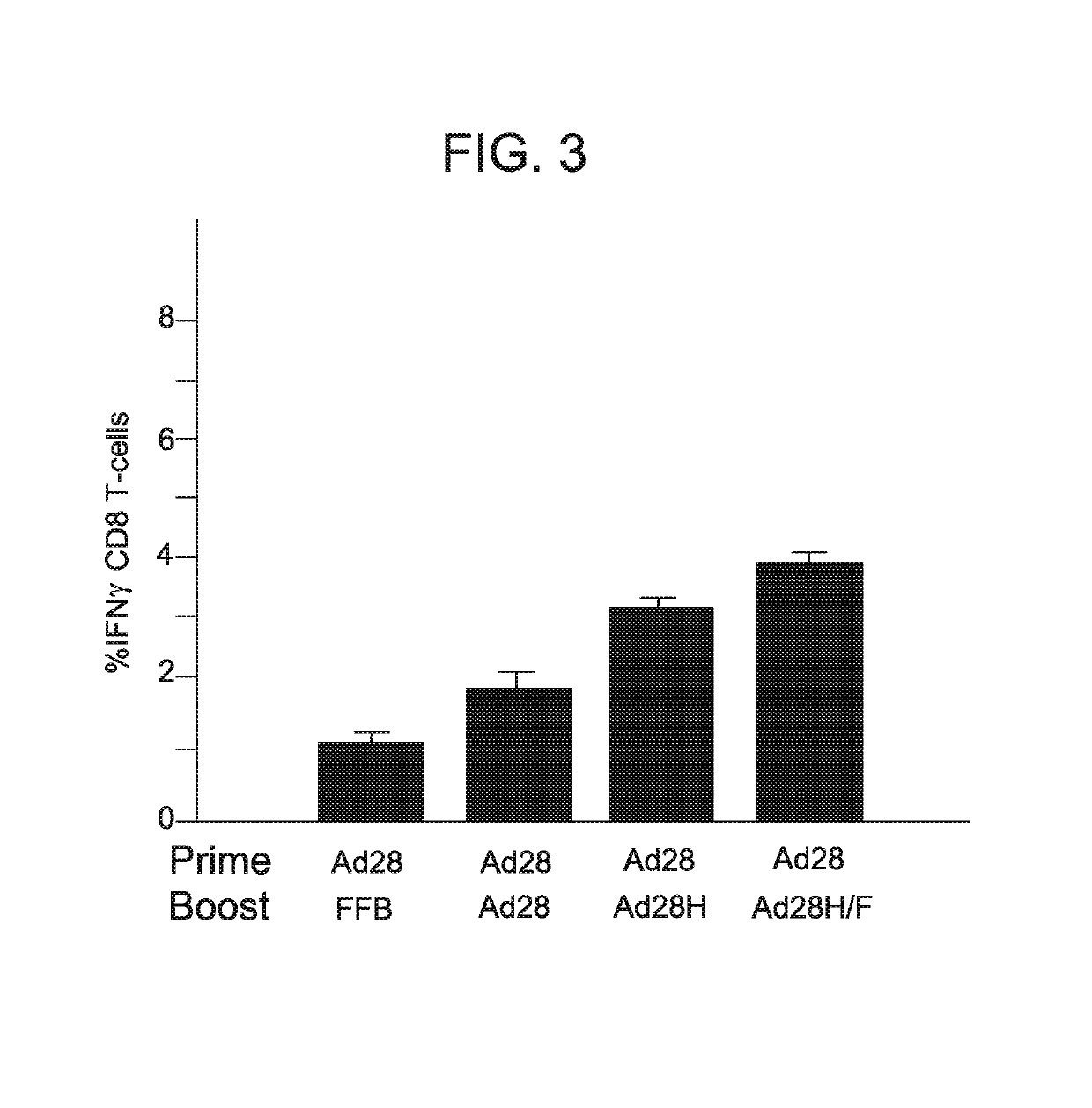
[0124]This example demonstrates that the administration of a vector comprising one or more nucleic acid sequences encoding one or more HSV antigens induces an immune response (T cell response) against HSV.
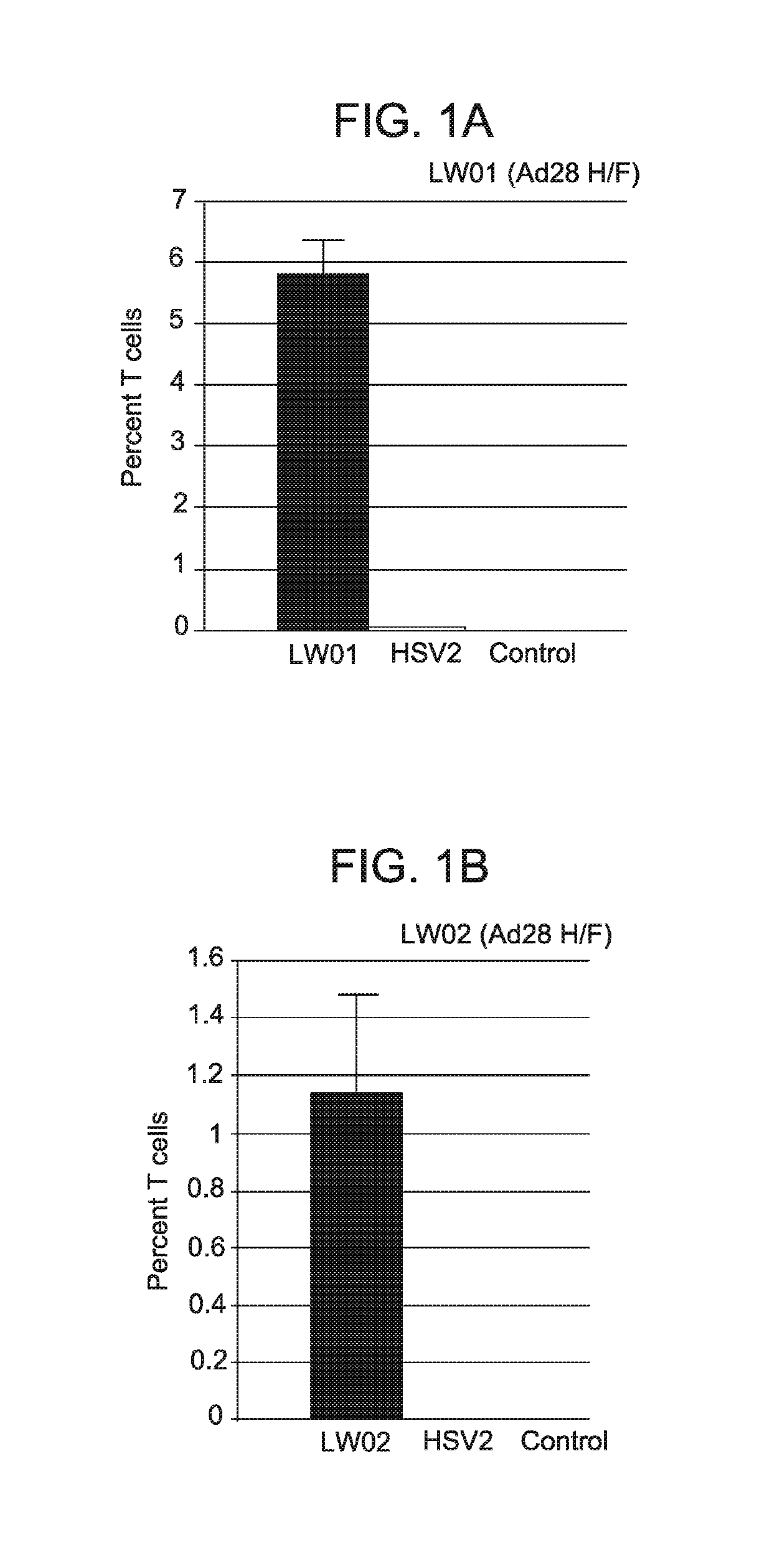
[0125]Two modified serotype 28 adenoviral vectors comprising hexon and fiber from a serotype 45 adenoviral vector (Ad28 H / F) were produced using the methods described in Example 1. The first vector comprised a nucleic acid sequence comprising SEQ ID NO: 7 (a wild-type (non-modified) UL47; designated LW01), while the second vector comprised a nucleic acid sequence comprising SEQ ID NO: 5 (inventive UL19 nucleic acid sequence; designated LW02).
[0126]T cell response in mice following a single intramuscular administration of each adenoviral vector-delivered antigen (1×109 PU) was compared to natural infection (1×106 PFU of HSV administered intravaginally) as described in Example 2. Animals infected with HSV showed symptoms of HSV infection. As a negative control, animals were injected ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com