Methods and Compositions for Treating Idiopathic Pulmonary Fibrosis
a technology of pulmonary fibrosis and compositions, applied in the direction of drug compositions, dispersed delivery, respiratory disorders, etc., can solve the problems of increasing the effort associated with breathing, slow decline of lung function, respiratory failure and death, etc., and achieve the effect of effective and safe manner
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Establishment of Positive and Negative Controls
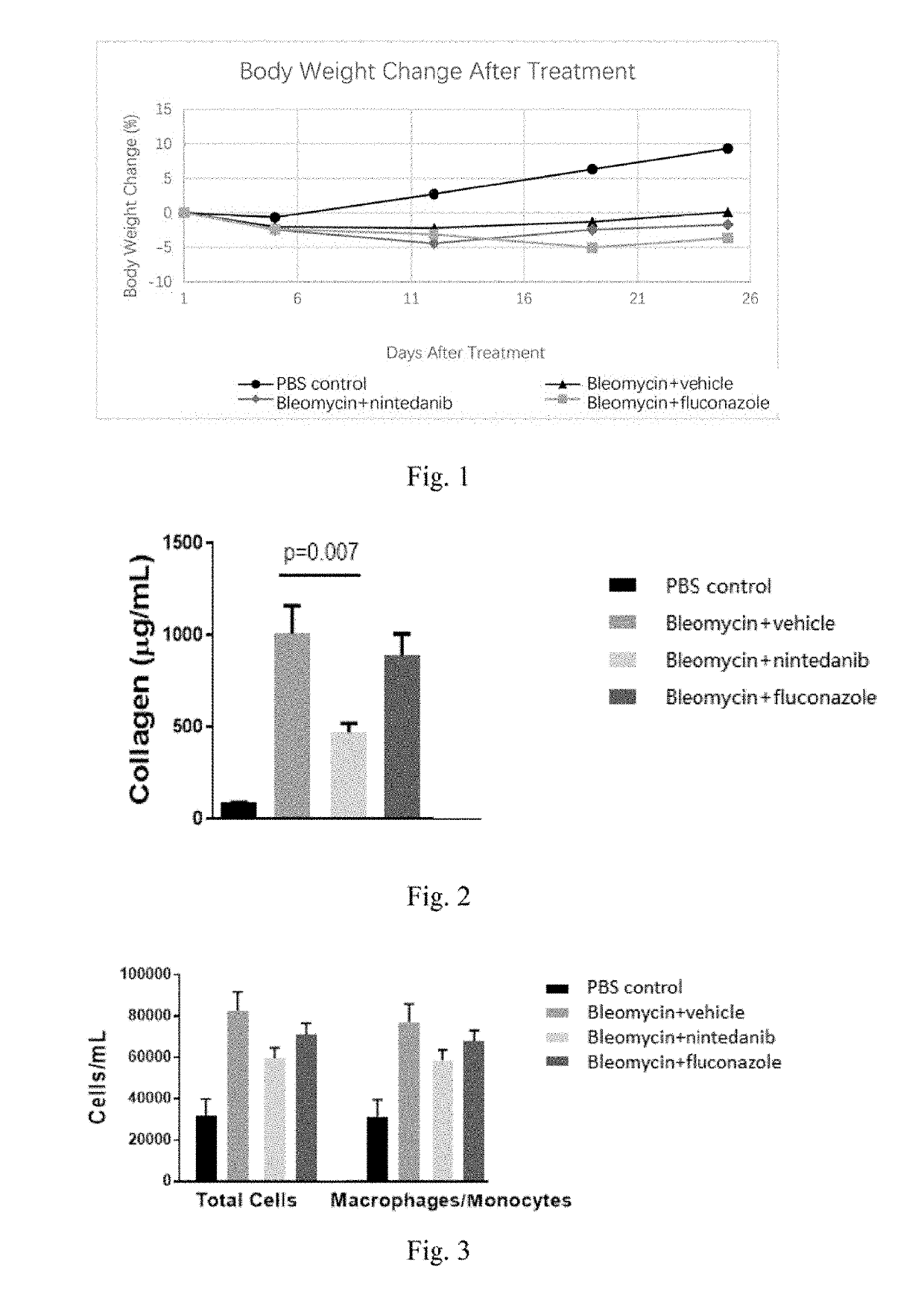
[0166]Forty male C57BL / 6 mice (Nanjing Biomedical Research Institute of Nanjing University) were randomly divided into two groups on Day 1, 10 animals for one group (referred to as Control group or Group 1) and 30 for the other. The animals in the Control group were administered intratracheally with PBS at a dose of 2 mL / kg while the others were administered intratracheally with bleomycin (Cat#HY-17565, MCE) at a dose of 0.66 mg / kg.
[0167]On Day 5, the mice with bleomycin treatment were divided into three groups at random (referred to as Group 2-4, n=10), and orally administered with vehicle (0.5% Methyl cellulose), nintedanib (Kangmanlin Co. Ltd., prepared in 0.5% Methyl cellulose with a final concentration of 6.0 mg / mL) and fluconazole (Kangmanlin Co. Ltd., prepared in 0.5% Methyl cellulose with a final concentration of 7.0 mg / mL) at daily doses of 10 mL / kg, 60 mg / kg and 70 mg / kg, respectively. The mice in the Control group was adminis...
example 2
Oral and Combination Treatment of Itraconazole Inhibited Fibrosis
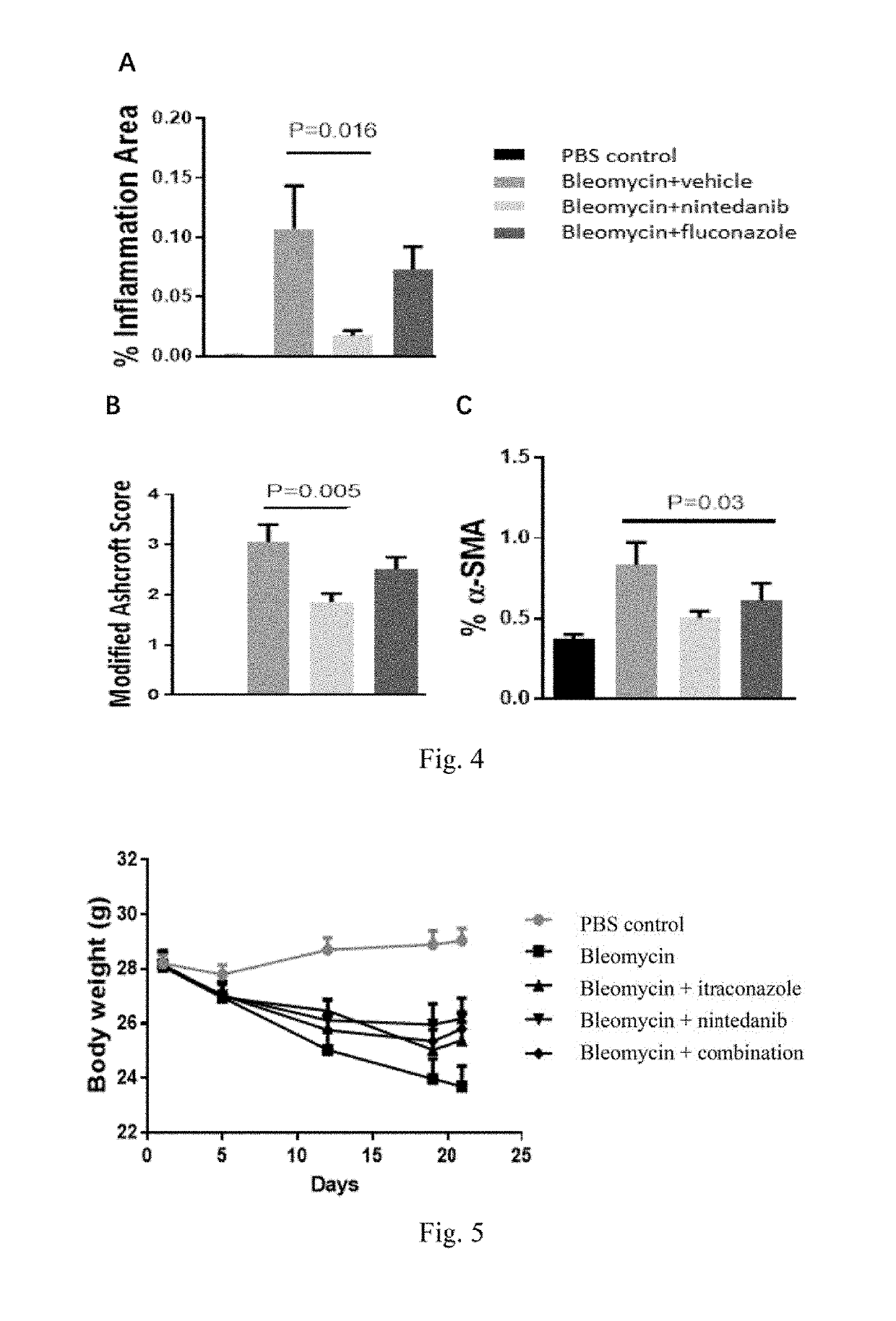
[0179]Fifty male C57BL / 6 mice (Gempharmatech Co., Ltd.) were randomly divided into two groups on Day 1, 10 animals for one group (referred to as Control group or Group 1) and 40 for the other. The animals in the Control group were administered intratracheally with PBS at a dose of 2 mL / kg while the others were administered intratracheally with bleomycin (Cat#HY-17565, MCE) at a dose of 0.66 mg / kg.
[0180]On Day 5, the mice with bleomycin treatment were divided into four groups at random (referred to as Group 2-5, n=10), and orally administered with vehicle (DMSO: PEG400=1:9, V / V), itraconazole (Kangmanlin Co. Ltd. prepared in DMSO / PEG400 with a final concentration of 1.5 mg / mL), nintedanib (Kangmanlin Co. Ltd., prepared in DMSO / PEG400 with a final concentration of 6.0 mg / mL), and itraconazole +nintedanib (prepared in DMSO / PEG400 with final concentrations of 1.5 mg / mL and 6.0 mg / mL) at daily doses of 10 mL / kg, 15 mg / kg, 6...
example 3
Inhalation Treatment of Itraconazole Inhibited Fibrosis
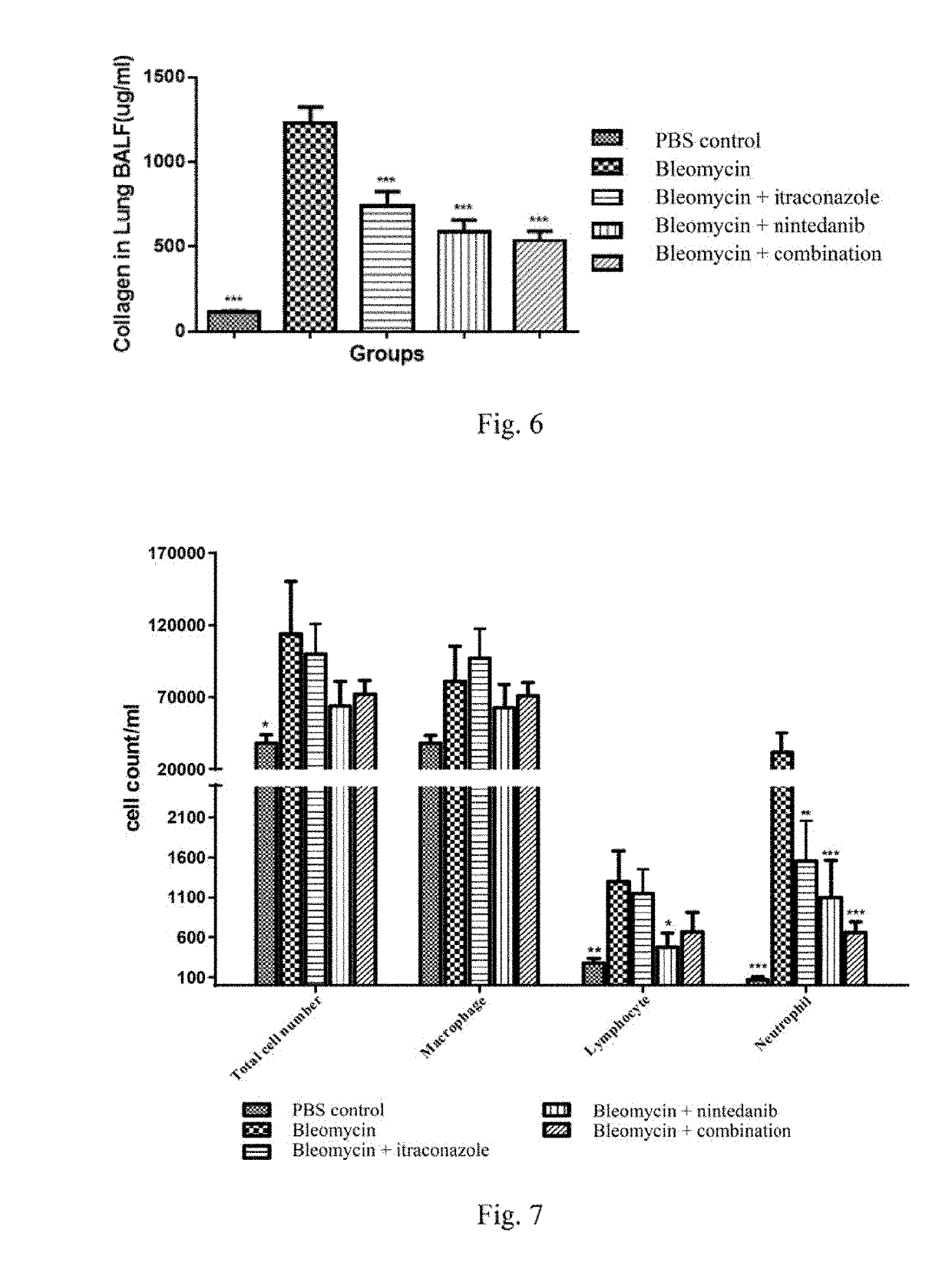
[0186]Sixty male ICR mice (SHANGHAI SLAC LABORATORY ANIMAL CO., LTD) were randomly divided into two groups on Day 1, 10 animals for one group (referred to as Control group or Group 1) and 50 for the other. The mice were anesthetized by intraperitoneal injection of 1.5% pentobarbital sodium solution at a dose of 0.1 mL / 20 g, and then supinely positioned and immobilized. Iodine was used to disinfect the neck hair and skin. Later, the neck skin was cut to expose the trachea. 50 μL of PBS or bleomycin hydrochloride (Hisun Pfizer pharmaceutical Co., LTD, 17001711) in 0.9% sodium chloride solution (0.35 USP / ml, i.e., 350 bleomycin units / mL) was quickly sprayed as mist into the trachea of mice from the Control Group or the other group with a high pressure syringe connected to a spraying nozzle. At the end, the skin was sutured and disinfected, and the mice were returned to the cages for recovery.
[0187]On Day 5, the mice treated with bl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com