Treatment of topical and systemic bacterial infections
a bacterial infection and topical technology, applied in the field of topical bacterial infections and systemic bacterial infections, can solve the problems of resistance strain emergence, lack of demonstration of efficacy, no working formulation or proof of principle, etc., to reduce the immune response, increase the likelihood of neutralising antibodies, and low stimulation of antibody responses.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Bacteriophage Formulations for Acne Treatment
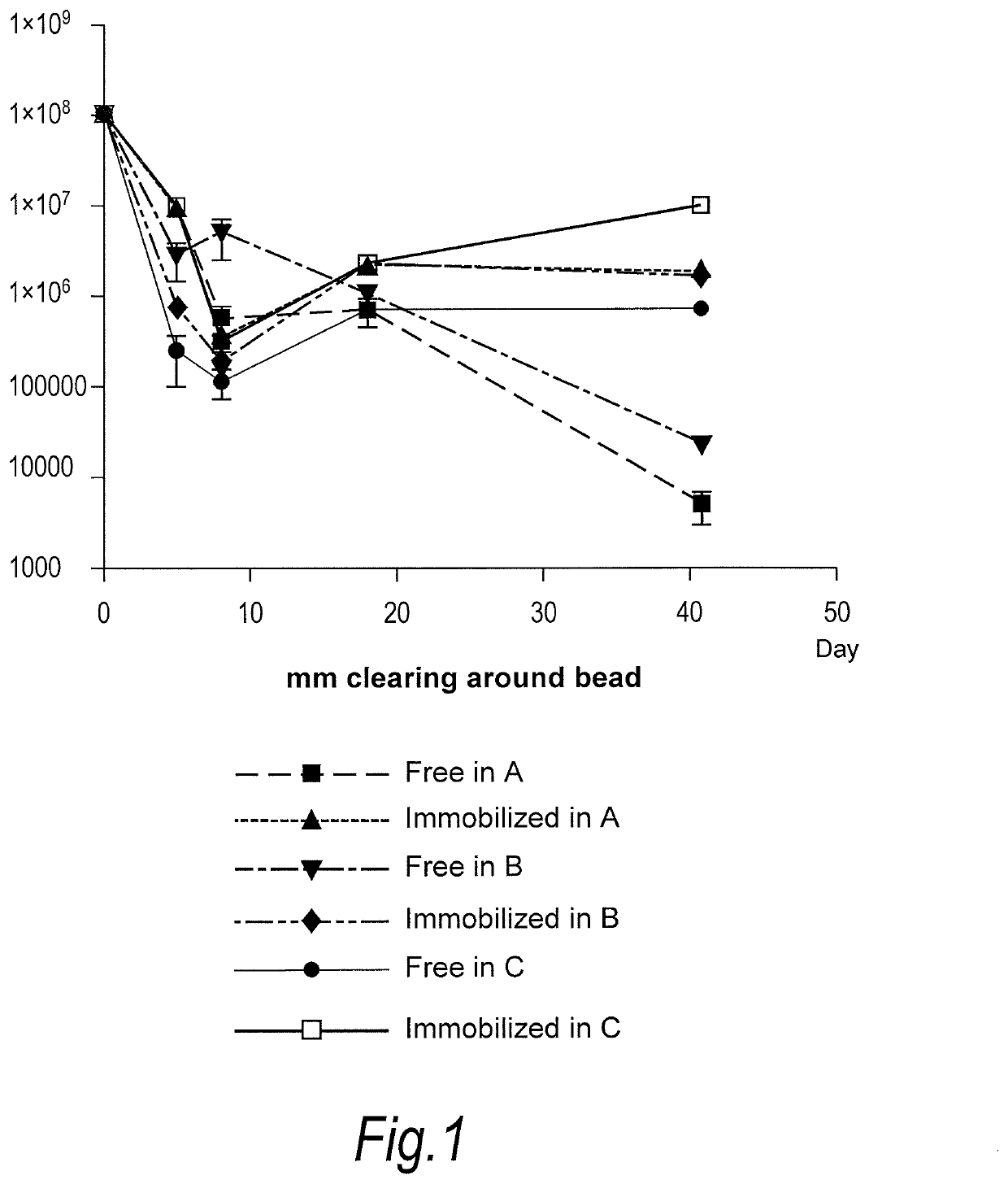
[0085]Propionibacterium acnes bacteriophages (FP pa1) were immobilised onto nylon beads (average diameter 10 microns) and mixed into Formulations A, B and C as set out below. Each Formulation was then tested for survival of bacteriophage at room temperature.
[0086]Formulation A—Aqueous Cream
Anhydrous Lanolin1.0%w / wWhite Soft Paraffin BP14.5%w / wLight Liquid Paraffin PhEur12.6%w / wWater[to 100%]
[0087]Formulation B—Face Wash (Commercially Available Under the Trade Mark “Clearasil”)[0088]Product contents: Aqua, Sodium Gluconate, Propylene Glycol, Octyldodecanol, Steareth-2, Cyclopentasiloxane, Steareth-21, Salicylic Acid, Cetyl Alcohol, Behenyl Alcohol, Cyclohexasiloxane, Polyacrylamide, C13-14 Isoparaffin, Xanthan Gum, Phenoxyethanol, Magnesium Aluminum Silicate, Laureth-7, Menthol, Methylparaben, Butylparaben, Ethylparaben, Isobutylparaben, Propylparaben, CI 77891.
[0089]Formulation C−Gel (Commercially Available Under the Trade Mark “Dr Spot”)...
example 2
Formulation for Topical Use (S. aureus)
[0096]Base Cream Preparation:
[0097]120 g of Emulsifying Ointment BP was heated to 60 degrees C. and mixed with 270 ml water also heated to the same temperature. The mixture was carefully stirred as it cooled, producing a smooth, white cream formulation. The cream was cooled to room temperature and divided into 5 equal portions.
[0098]Bacteriophage-Particle Production:
[0099]Nylon 12 particles of average diameter 3 microns were treated by corona discharge (75 kV field) and rapidly added to a bacteriophage suspension at 1×109 pfu / ml. Particles were washed 3 times to remove non-bound bacteriophages. Using this method, 5 separate 2 ml preparations were made utilizing one of each of 5 different strains of bacteriophage specific for S. aureus from stored phage stock.
[0100]Formulation:
[0101]To each of the 5 separate portions of cream base was added a separate bacteriophage-particle preparation by admixture and agitation until the suspension had been ful...
example 3
Formulation for Topical Use (P. acnes)
[0102]A formulation was prepared as follows.[0103]Oil phase: Stearic acid 4%, stearyl alcohol 5%, lanolin 7%, isopropyl myristate 8%.[0104]Aqueous phase: Methyl cellulose 1%, in purified water
[0105]The two phases were prepared separately by weight and heated to 70° C. The water phase was then mixed with the oil phase by trituration till the cream congealed and cooled.
[0106]Nylon 6,6 particles of average diameter 10 microns were treated by passing through a corona discharge at 70 kv and rapidly added to a mixed bacteriophage preparation containing 5 different bacteriophages against P acnes at a final concentration of 1×109 pfu / ml. The particles were washed 3 times to remove non-bound bacteriophages and added to the cream base to give a final bacteriophage concentration of 1×105 pfu / ml.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com