Method for treating epstein-barr virus - positive cancer with immunotherapy
a technology of epstein-barr virus and immunotherapy, which is applied in the field of immunotherapy for epstein-barr virus-positive cancer, can solve the problems of limited treatment options for metastatic disease, limited use of npc, and inability to identify reliable biomarkers to predict the efficacy of checkpoint inhibitors, etc., and achieve the effect of accurately identifying one- and two-year survivors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
tal Procedures
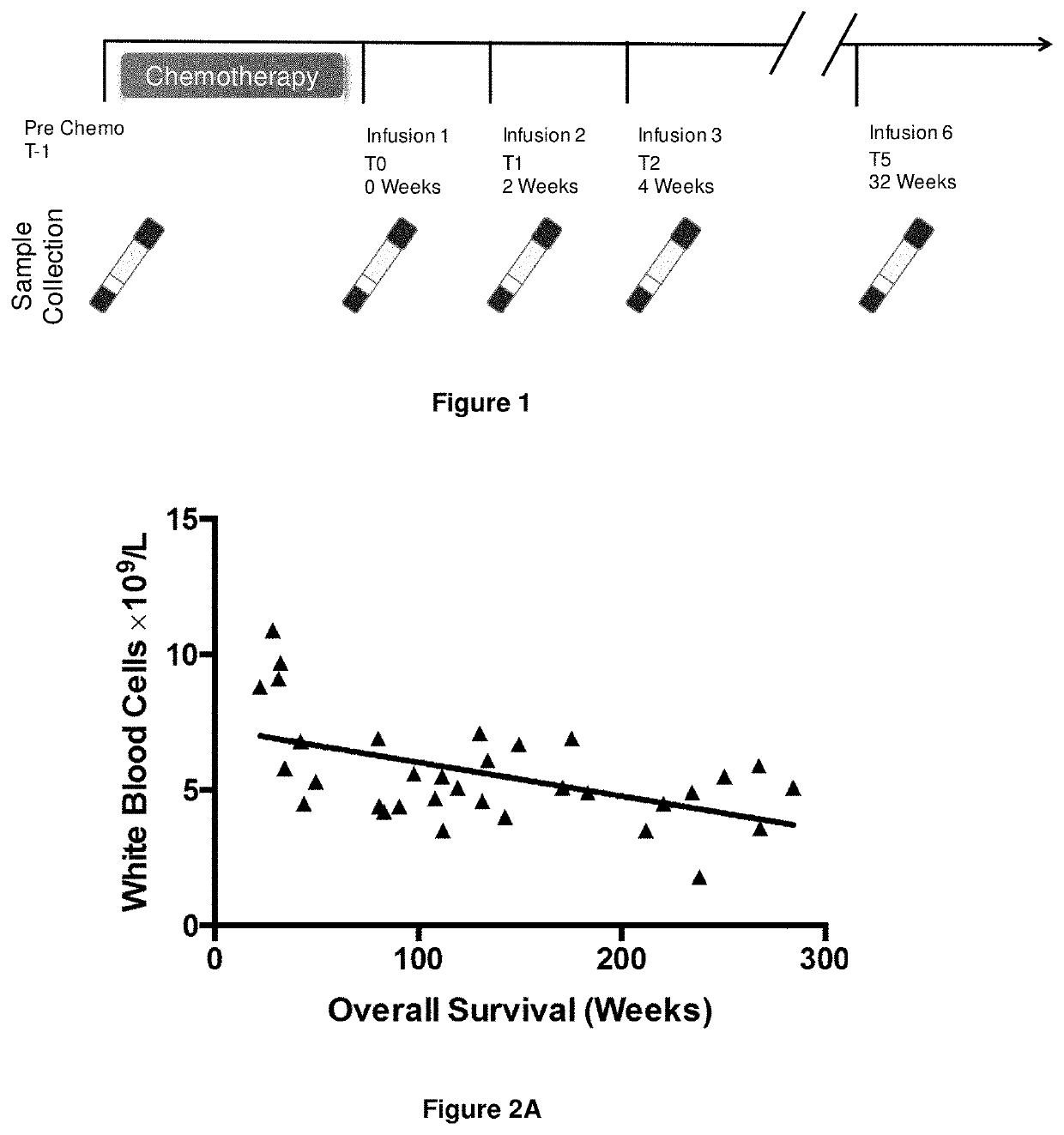
[0365]1.1 Immunotherapy Trial and Sample Collection
[0366]A Phase II clinical trial was performed in which Epstein-Barr virus (EBV)-positive nasopharyngeal carcinoma (NPC) patients were treated with gemcitabine and carboplatin for four cycles, followed by treatment by adoptive transfer of autologous, in vitro expanded, EBV-specific CD8+ T-cells.
[0367]The results demonstrated unprecedented efficacy. The two-year overall survival rate was 62.9%, and the three-year overall survival rate was 37.1%. These two- and three-year overall survival rates are amongst the best survival rates for treatment of advanced NPC.
[0368]Analysis of the Phase II trial data showed that there was a positive correlation between long-term survivors and the ability of their CTLs to produce IFN-γ in response to LMP2 antigen presentation.
[0369]Throughout the immunotherapy trial peripheral blood was sampled from the patients. The time points examined were:[0370]Pre chemotherapy (T−1);[0371]Post chemoth...
example 2
Survivors Experience Increased Lymphoid but Decreased Myeloid Numbers Post CTL Immunotherapy
[0392]Throughout the immunotherapy trial peripheral blood was sampled from the patients. In order to assess the impact of immunotherapy analysis was focused on the time point two weeks after the first immunotherapy infusion (T1; see FIG. 1).
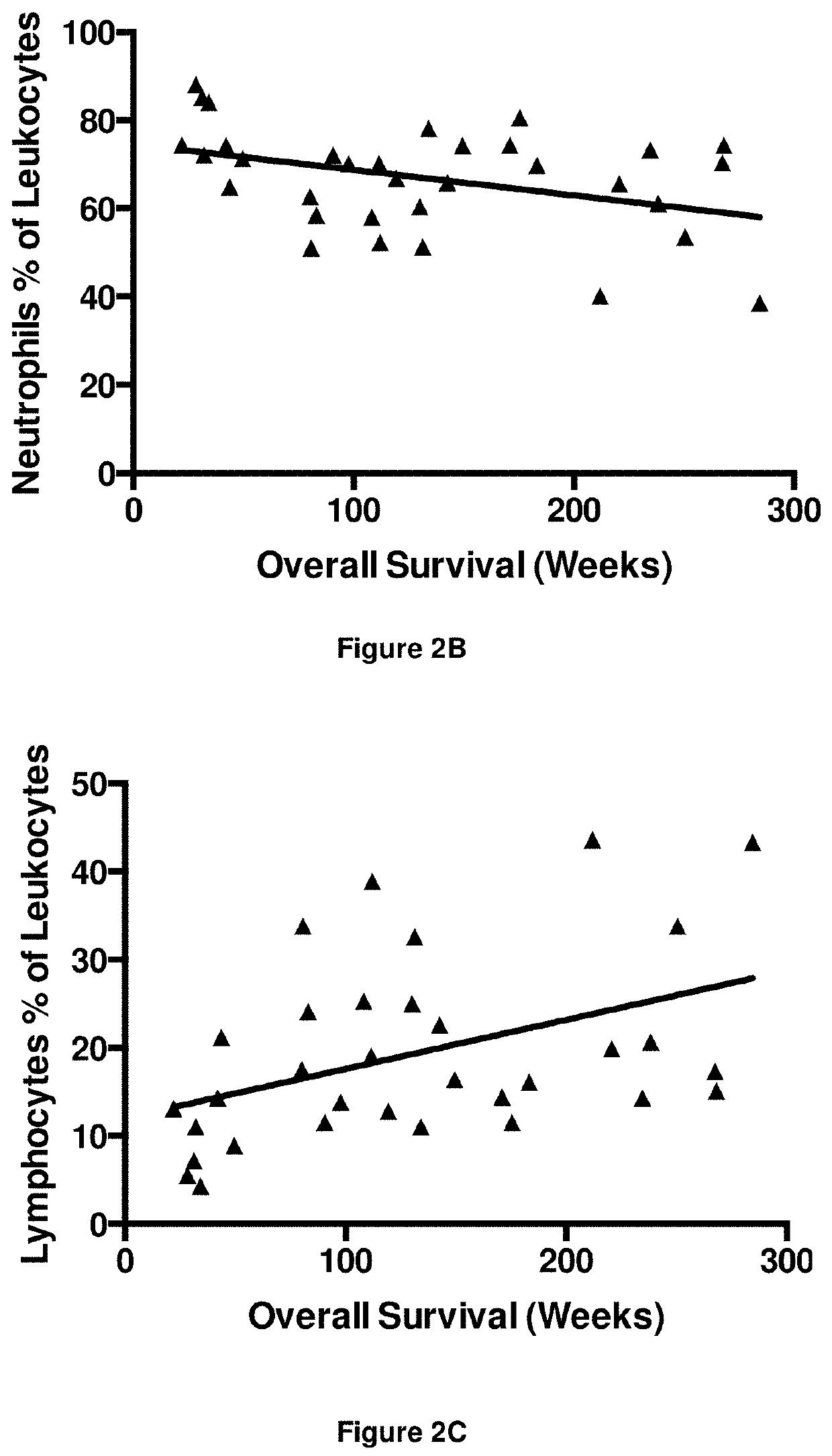
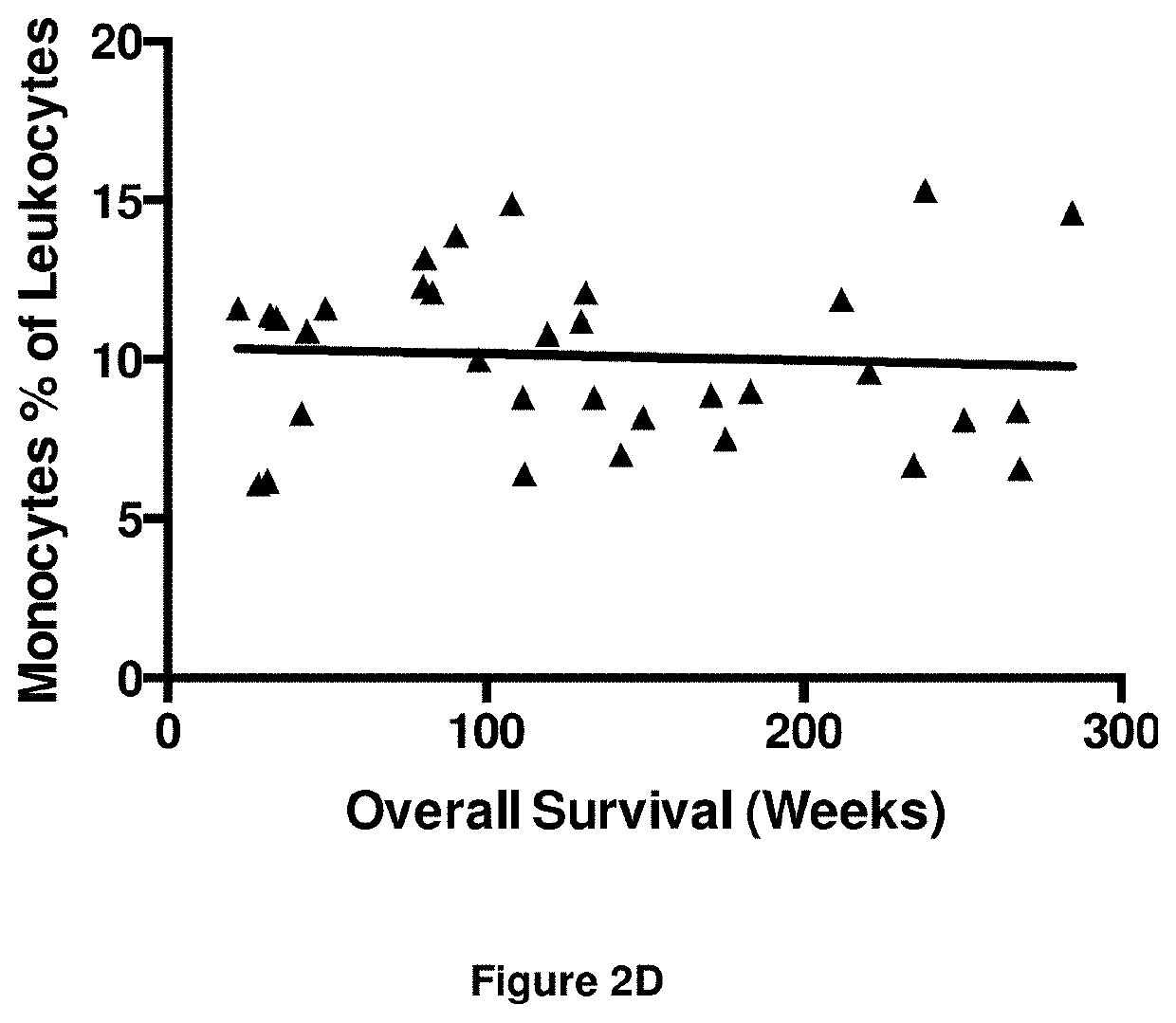
[0393]The numbers of peripheral blood leukocytes at time point T1 were assessed to detect any gross changes in the immune system as a result of immunotherapy. The results are shown in FIGS. 2A to 2E.
[0394]There was a significant negative correlation between the number of leukocytes and overall survival (r=−0.43; FIG. 2A). Neutrophil numbers (as a proportion of leukocytes) were also significantly, negatively associated with survival, albeit with poor correlation (r=−0.32; FIG. 2B). Conversely there was a significant positive correlation between lymphocyte numbers (as a proportion of leukocytes) and overall survival (r=0.46; FIG. 2C). No correlation was obse...
example 3
l CTL Immunotherapy Results in Increased IFNγ
[0396]In order to assess whether or not the increase in lymphocyte numbers was also influencing viral load and cytokine production, patient sera was analysed for cytokine production by luminex assay, and EBV DNA was quantified by qPCR. The results of the analyses are shown in FIG. 4A to 4F. The data shown in FIGS. 4A to 4D, 5, 6 and 7A and 7B is based on analysis of serum obtained from samples isolated at time point T1, i.e. two weeks after the first infusion of EBV-specific CTL.
[0397]Within two weeks after the first immunotherapy infusion there was a significant positive correlation between IFNγ production and survival (FIG. 4A). Prior to immunotherapy (i.e. at T0), there was no correlation was observed between survival and IFNγ concentrations in the sera (Table 2, FIG. 5). Conversely, survival was strongly correlated with a significant decrease in EBV viral load (FIG. 4B). Production of myeloid-expressed chemokines such as CCL20 was sim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com