Derivatives of amanita toxins and their conjugation to a cell binding molecule
a technology of amanita toxins and cell binding molecules, which is applied in the field of cytotoxic agents and derivatives of amanita toxins, can solve the problems of low yield from isolation and fermentation, and the field is incredibly challenging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
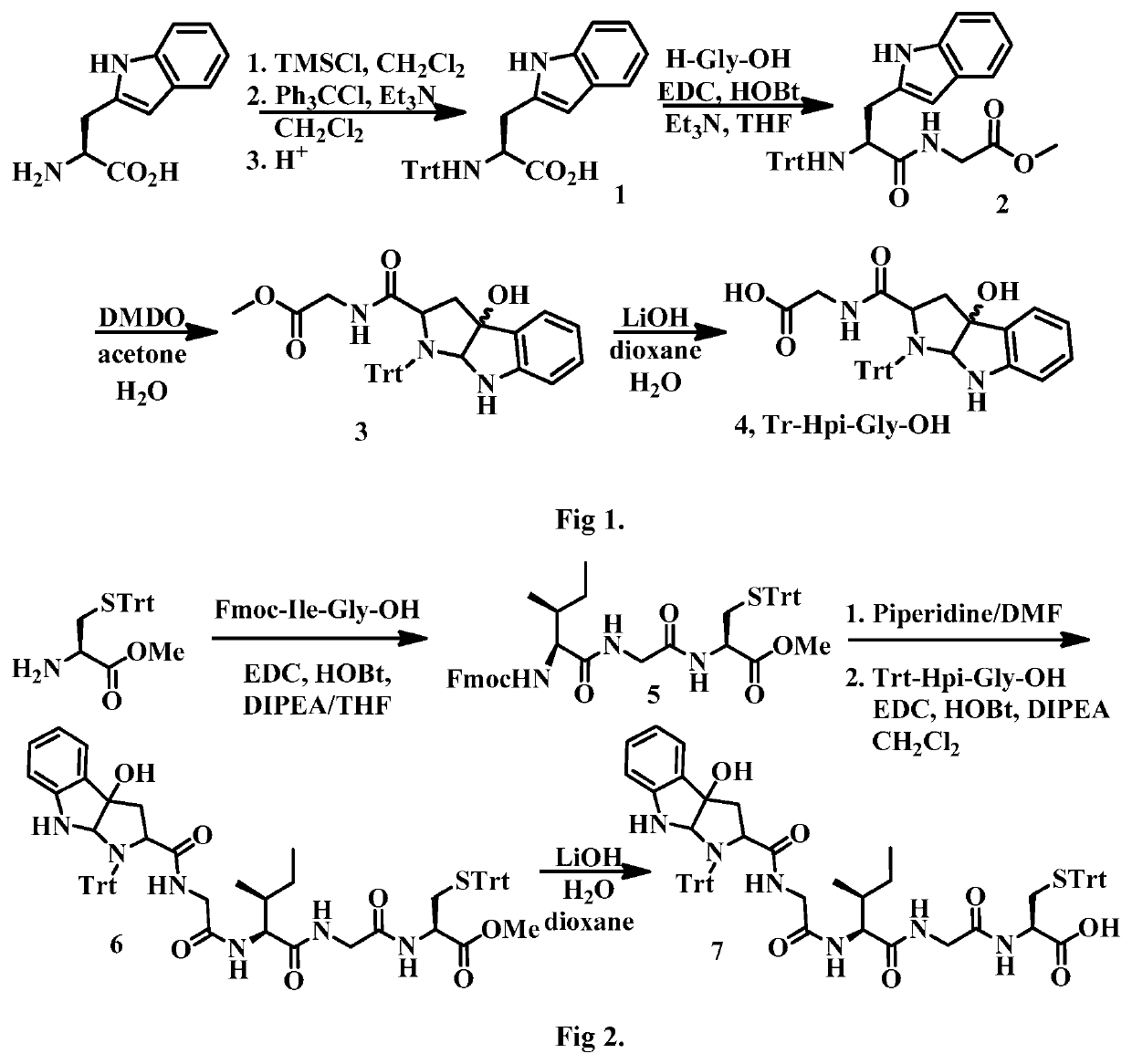
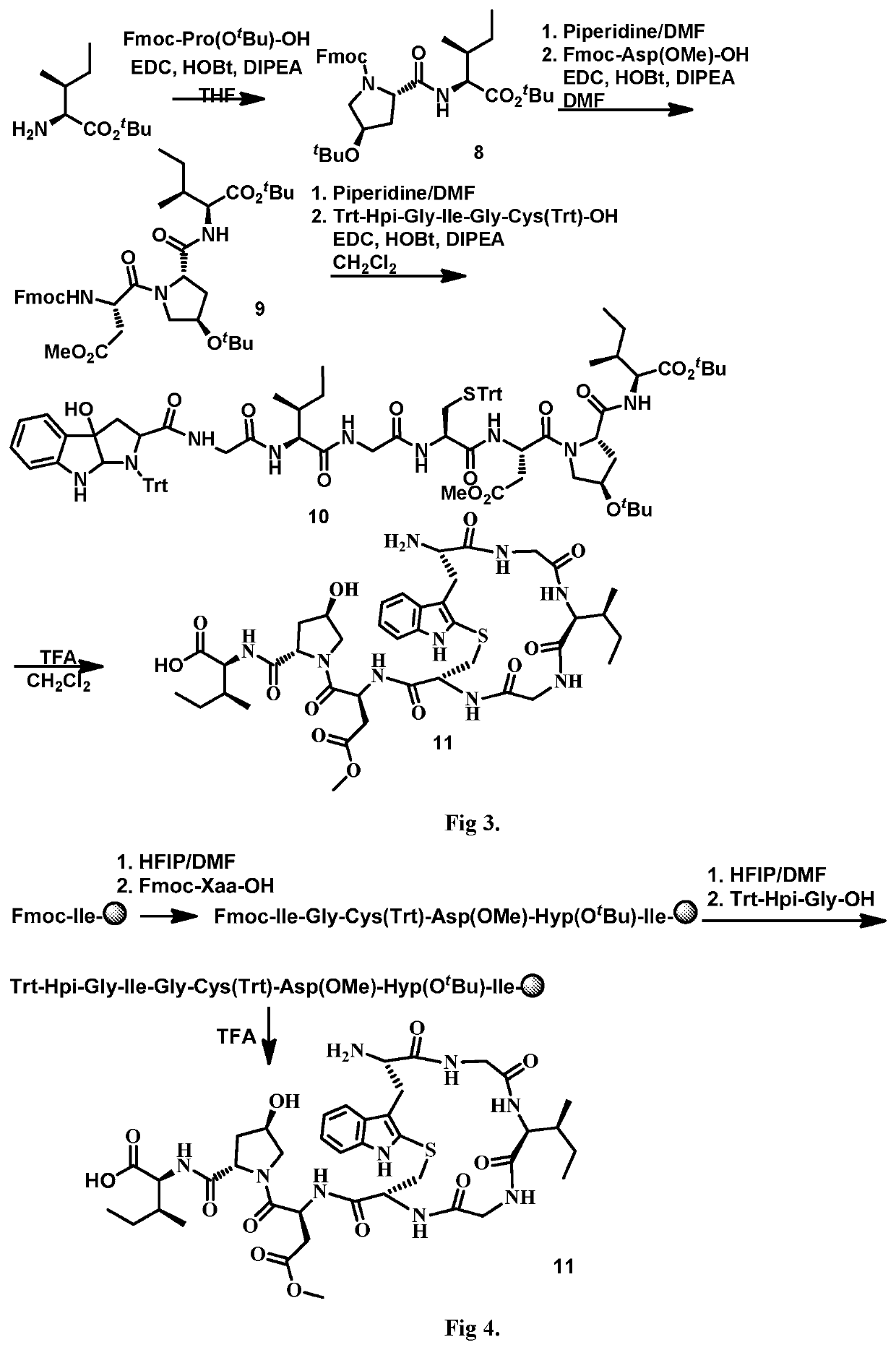
Synthesis of (S)-3-(1H-indol-2-yl)-2-(tritylamino)propanoic acid (1)
[0201]
[0202]Chlorotrimethylsilane (3.4 mL, 26.9 mmol) was added slowly to a suspension of L-tryptophan (5.00 g, 24.5 mL) in methylene chloride (40 mL) at r.t. The mixture was continuously stirred for 4.5 h and triethylamine (6.8 mL, 49.0 mmol) was added, followed by a solution of triphenylmethyl chloride (7.17 g, 25.7 mmol) in methylene chloride (20 mL). The mixture was stirred at r.t. for 20 h and then quenched with methanol (25 mL). The reaction was concentrated to near dryness and re-dissolved in methylene chloride, washed with 5% citric acid solution (3×) and brine. The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated. The residue was further dissolved in methylene chloride and filtered over a celite pad and the filtrate was concentrated to give a pale white foam (11.8 g), which was used directly in the next step. ESI MS m / z 446.5 ([M+H]+).
example 2
Synthesis of (S)-methyl 2-(3-(1H-indol-2-yl)-2-(tritylamino)propan amido)acetate (2)
[0203]
[0204]To a solution of acid (9.27 g, 30.7 mmol) in THF (30 mL) was added glycine methyl ester hydrochloride (2.85 g, 22.8 mmol) and HOBt (3.08 g, 22.8 mmol). The mixture was cooled to 0° C. and triethylamine (7.4 mL, 51.9 mmol) was added, followed by EDC.HCl (4.38 g, 22.8 mmol) in portions. The mixture was allowed to warm to r.t. and stirred for 20 h and then concentrated and redissolved in methylene chloride and washed with 5% citric acid solution (3×) and brine. The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated. The residue was triturated with ethyl acetate and a white solid was collected by filtration (6.46 g, 65% yield over two steps). ESI MS m / z 518.2 ([M+H]+).
example 3
Synthesis of Dimethyldioxirane (DMDO)
[0205]Distilled water (20 mL), acetone (30 mL), and NaHCO3 (24 g, 0.285 mol) were combined in a 1-L round-bottomed flask and chilled in an ice / water bath with magnetic stirring for 20 min. After 20 min, stirring was halted and Oxone (25 g, 0.0406 mol) was added in a single portion. The flask was loosely covered and the slurry was stirred vigorously for 15 min while still submerged in the ice bath. The flask containing the reaction slurry was then attached to a rotary evaporator with a bath at room temperature. The bump bulb (250 mL) was chilled in a dry ice / acetone bath and a vacuum of 165 mtor was applied via a benchtop diaphragm pump. After 15 min, the bath temperature was raised to 40° C. over 10 min. When the bath reached 40° C., the distillation was halted immediately via releasing the vacuum and raising the flask from the heated water bath. The pale yellow acetone solution of DMDO was decanted from the bump bulb directly into a graduated cy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| apparent equilibrium dissociation constant Kd | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com