Pdl1 peptides for use in cancer vaccines
a cancer vaccine and peptide technology, applied in the field of pdl1 peptide fragments, can solve the problems of malignant cancer, poor prognosis for affected individuals, and the inability of the immune system to respond effectively to these antigens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
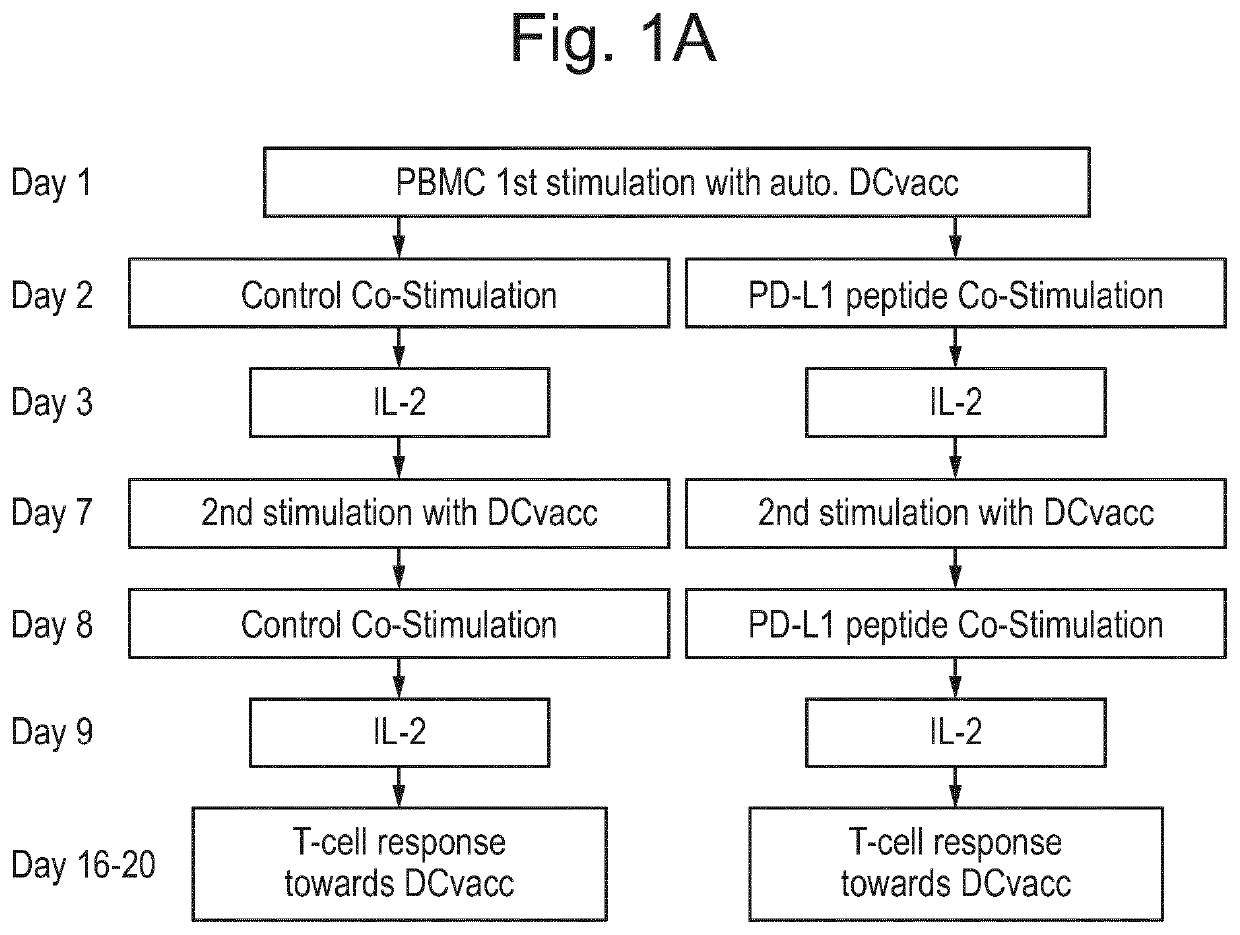
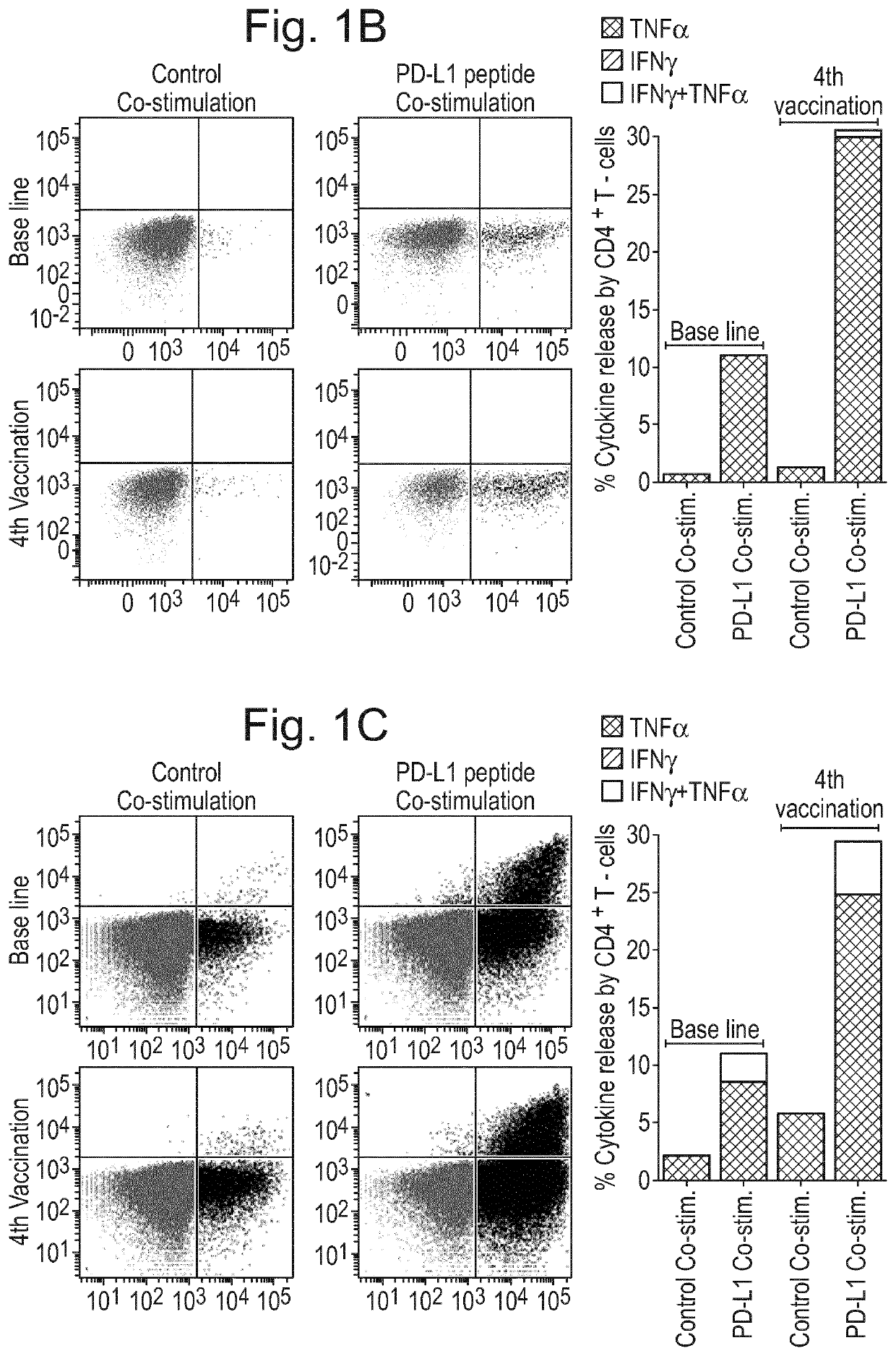
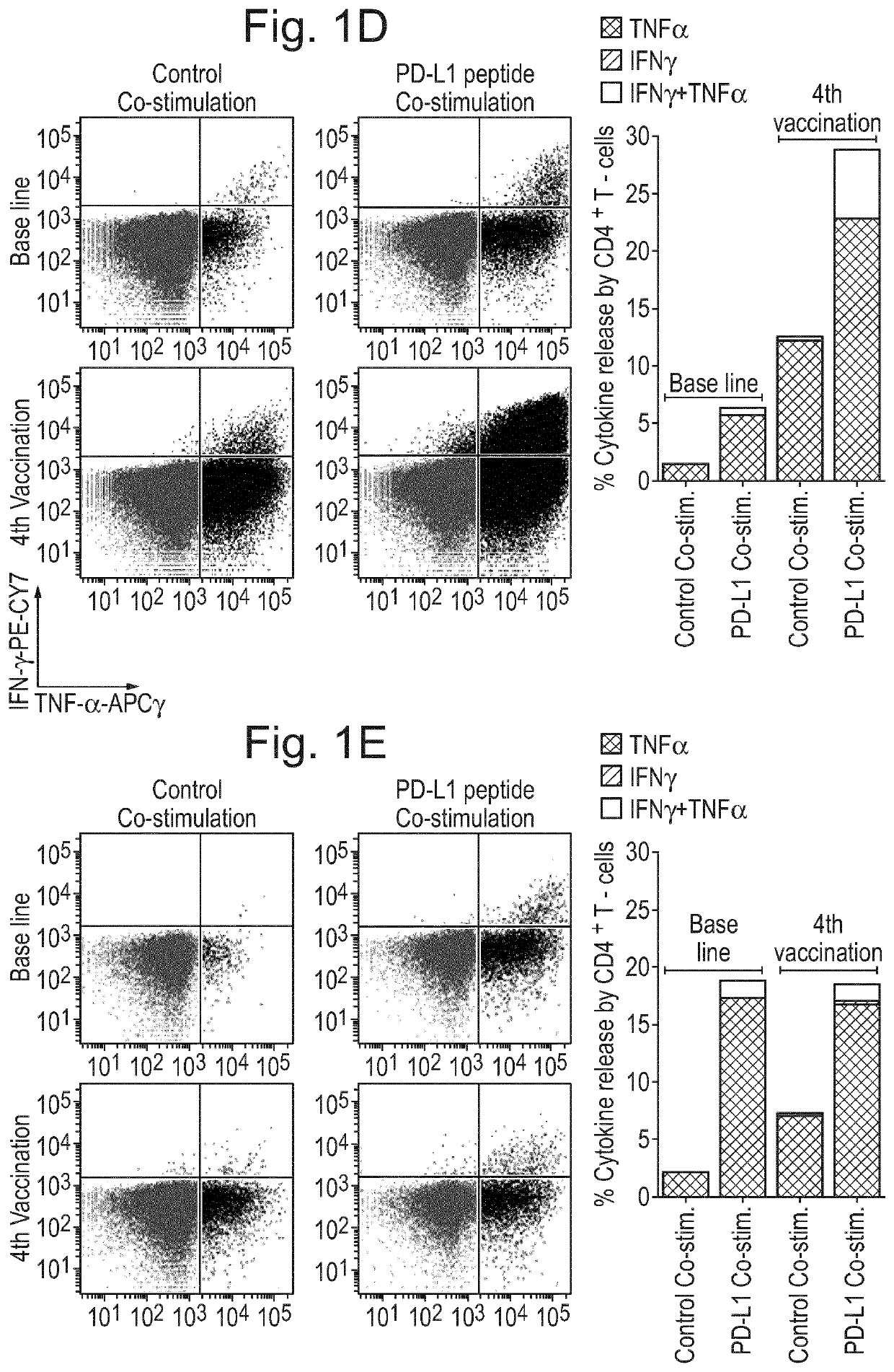
[0223]Patients and Donors 26 stage IV melanoma patients were enrolled in an open-labeled, non-randomized phase I / II study (EudraCT number 2009-010194-20; Clinicaltrials.gov identifier: NCT00978913). The protocol was approved by the Scientific Ethics Committee for The Capital Region of Denmark (H-A-2009-013), the Danish Medicines Agency (2612-4030), the Danish Data Protection Agency and conducted in accordance with the provisions of the Declaration of Helsinki. Written informed consent from the patients was obtained before study entry. The clinical and immunological results will be reported elsewhere (Borch et al., in preparation). In short patients were injected with autologous DC vaccines intradermally fortnightly six times and subsequently every four weeks until progression. Concomitantly, patients were treated with a metronomic cyclophosphamide regimen (50 mg twice a day) biweekly.
[0224]For immune monitoring purposes Peripheral Blood Mononuclear Cells (PBMC) ...
example 2
Spontanous Immune Responses Against IO104.1 in Human Patients
[0243]We first analysed the immune responses against two versions of IO104.1 in Tumor infiltrating T cells from melanoma patients. Next we analysed if PDL111 specific T cells were able to recognize IO104.1
Materials and Methods
Peptides
[0244]
(minimal epitope - SEQ ID NO: 92)PDL111 = CLGVALTFI(SEQ ID NO: 92)IO104 (PDLong2) = VILGAILLCLGVALTFIFRLRKG(SEQ ID NO: 52)IO104.1-OH = Arg-Thr-His-Leu-Val-Ile-Leu-Gly-Ala-Ile-Leu-Leu-Cys-Leu-Gly-Val-Ala-Leu-Thr-Phe-Ile-Phe-Arg-Leu-Arg-Lys-Gly-Arg-OH (C-terminus acid)(SEQ ID NO: 52 with C terminal amide)IO104.1-NH2 = Arg-Thr-His-Leu-Val-Ile-Leu-Gly-Ala-Ile-Leu-Leu-Cys-Leu-Gly-Val-Ala-Leu-Thr-Phe-Ile-Phe-Arg-Leu-Arg-Lys-Gly-Arg-NH2 (C-terminus amide)
[0245]The ELISPOT technique enabled screening a high number of peptide antigens for T-cell recognition, despite the availability of relatively few T-cells. We used the ELISPOT assay to quantify peptide-specific, effector cells that...
example 3
PDL1 Specific T Cells are Naturally Occurring in Mice
[0249]We hypothesized that if PD-L1 specific T cells are natural occurring, they should activate and expand in response to inflammation.
Materials and Methods
[0250]C56BL / 6 mice were injected with 1 μg IFNγ in 200 μl PBS i.p (or no injection for control) two days apart (day 0+2) to simulate inflammation. On day 5 the mice were sacrificed and a single cell solution of the removed spleen was made for further analysis by IFNγ-Elispot.
[0251]9×105 splenocytes / well were stimulated ex vivo in Elispot plates with 5 μg / ml peptides from murine (m)PDL1 for 18-20 hours. Spot count for the peptide stimulated wells was subtracted the background (spot count of wells with no peptide stimulation).
[0252]The peptides from PDL1 were selected based on the following reasoning.
[0253]The Sequence of mPD-L1 is:
(SEQ ID NO: 95 - N-terminal / signal sequence inbold, C-terminal / membrane-spanning regionunderlined)MRIFAGIIFT ACCHLLRAFT ITAPKDLYVV EYGSNVTMECRFPVEREL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight % | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| compositions | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com