Treatment of exercise-induced hypoglycemia in type 1 and insulin using type 2 diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
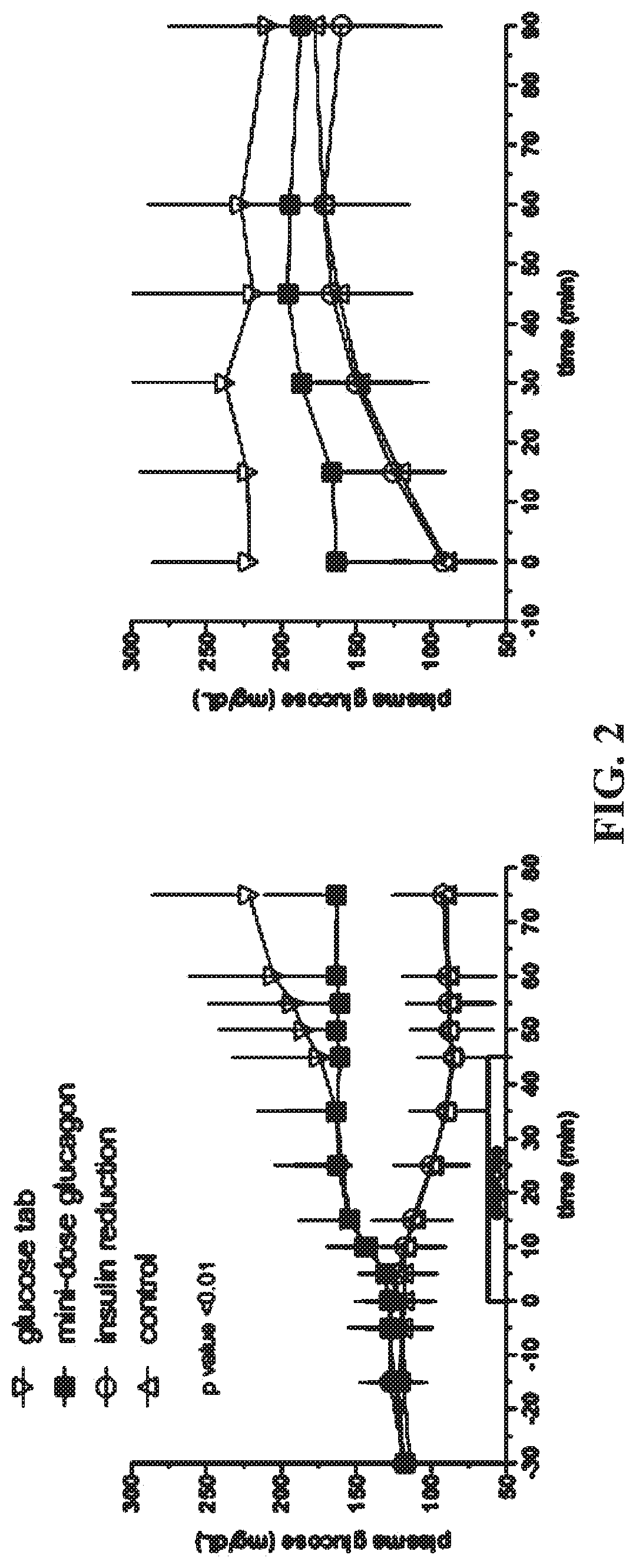
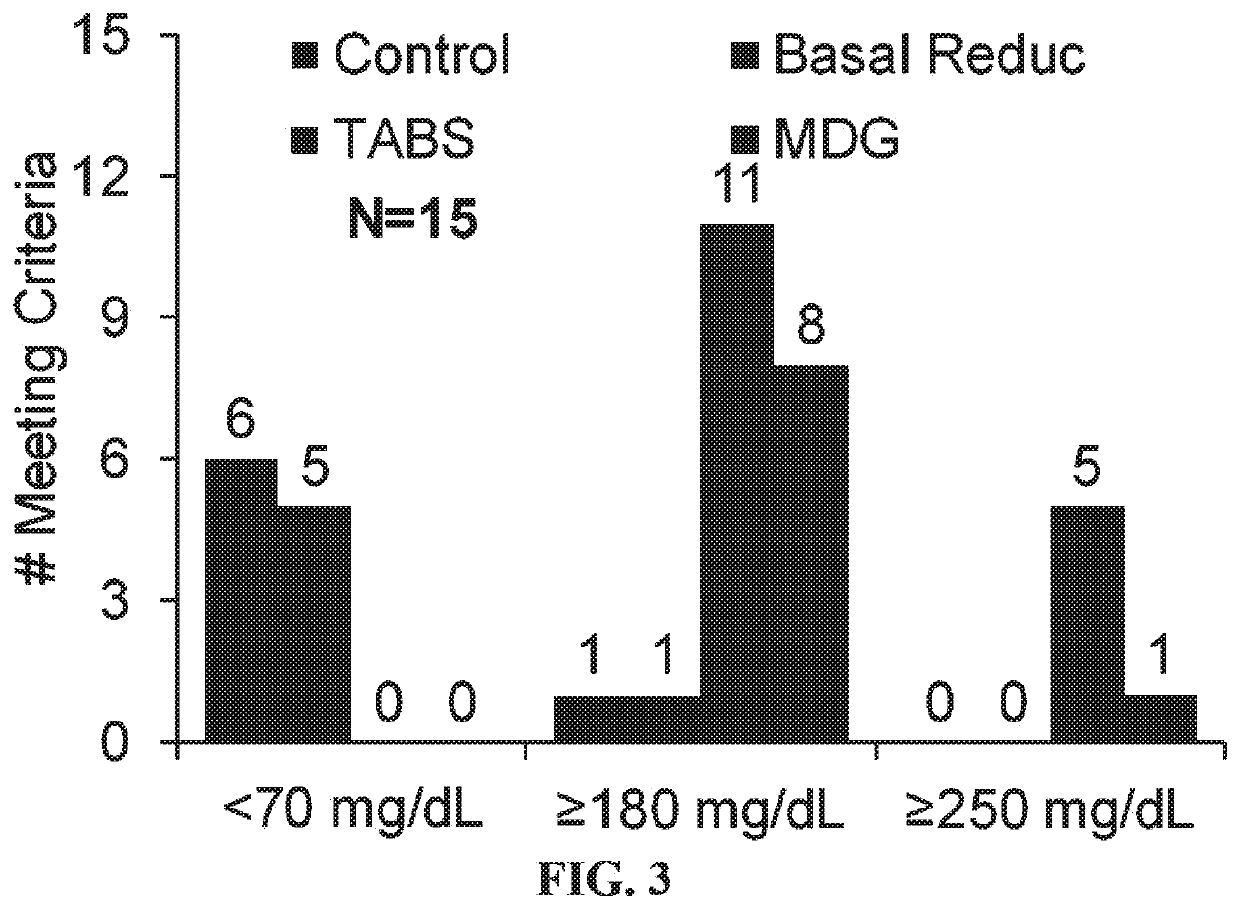
Use of Mini-Dose Glucagon to Prevent Exercise—Induced Hypoglycemia in Type 1 Diabetes (T1D)
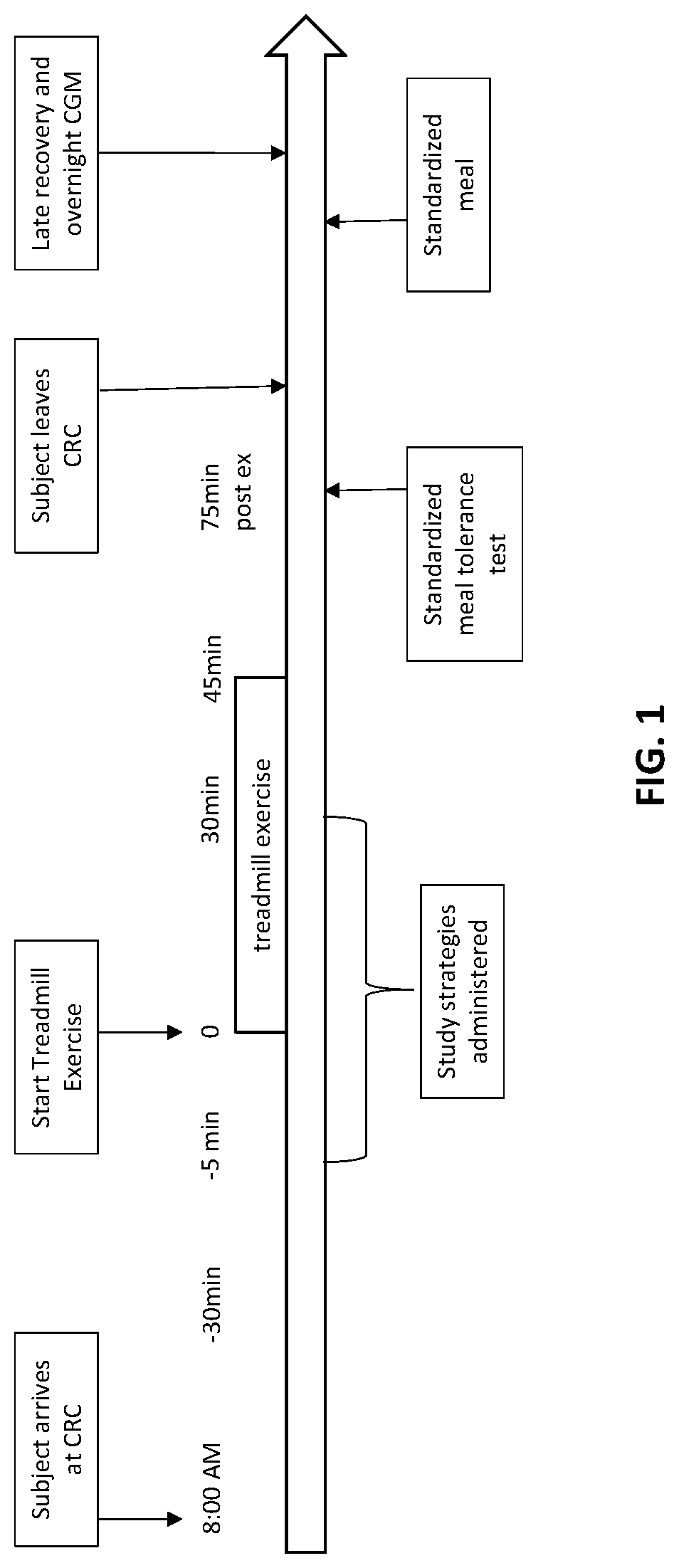
[0091]An example of one study to assess the effectiveness of mini-dose glucagon in the prevention or amelioration of exercise-induced hypoglycemia in type 1 diabetes (T1D) can include a randomized, 4-way crossover trial.
[0092]Visit Schedule. (1) Screening / Baseline Visit. This visit will be used to assess eligibility and can include determination of VO2 max for fitness evaluation and for the determination of exercise intensity for the study. (2) Randomized Crossover Trial. Each participant will undergo four aerobic exercise sessions (in random order), with different strategies for glucose regulation that include: (i) Control: Fasted exercise, no basal insulin reduction; (ii) Strategy 1: Fasted exercise, basal insulin reduction only (50% reduction in basal rate five minutes before exercise, for the duration of the exercise); (iii) Strategy 2: Fasted exercise, no basal adjustment+pre-exercise glu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com