Soluble alpha-klotho proteins, protein fragments, and uses thereof
a technology of soluble alpha-klotho proteins and protein fragments, which is applied in the field of modified soluble klotho proteins and isolated fragments of soluble klotho proteins, can solve the problems of inability to achieve parenteral regimens for chronic disorders, adverse effects of phosphate homeostasis on essentially every major tissue/organ, and insufficient replacement therapy alone. , to achieve the effect of suppressing egr1 transcription, promoting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
l Basis for αKlotho Co-Receptor Function
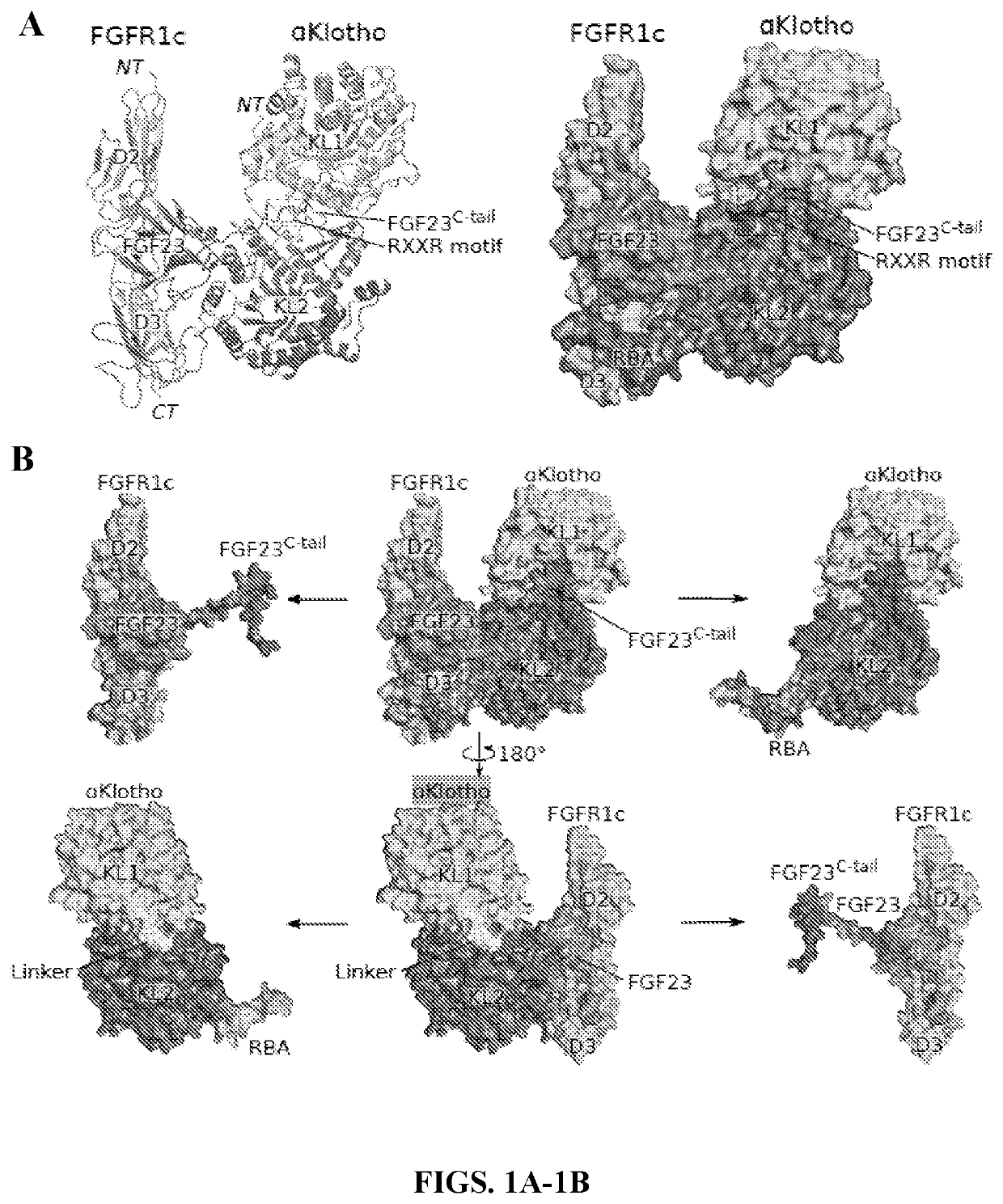
[0178]The crystal structure of a human 1:1:1 FGF23-FGFR1cecto-αKlothoect® ternary complex at 3.0 Å resolution was solved (Table 5). In this complex, αKlothoecto serves as a massive scaffold, tethering both FGFR1c and FGF23 to itself. In doing so, αKlothoecto enforces FGF23-FGFR1c proximity and thus augments FGF23-FGFR1c binding affinity (FIG. 1). The overall geometry of the ternary complex is compatible with its formation on the cell surface (FIG. 7A).
[0179]The binary FGF23-FGFR1cecto complex adopts a canonical FGF-FGFR complex topology in which FGF23 is bound between the receptor's D2 and D3 domains, engaging both these domains and a short interdomain linker (FIG. 8A). However, compared to paracrine FGFs, FGF23 makes fewer / weaker contacts with the D3 domain and D2-D3 linker, explaining the inherently low affinity of FGF23 for FGFR1c (FIGS. 8B-8C). Notably, analysis of the binding interface between FGF23 and FGFR1c D3 in the crystal structure ...
example 3
nterface Between αKlotho and FGFR1c
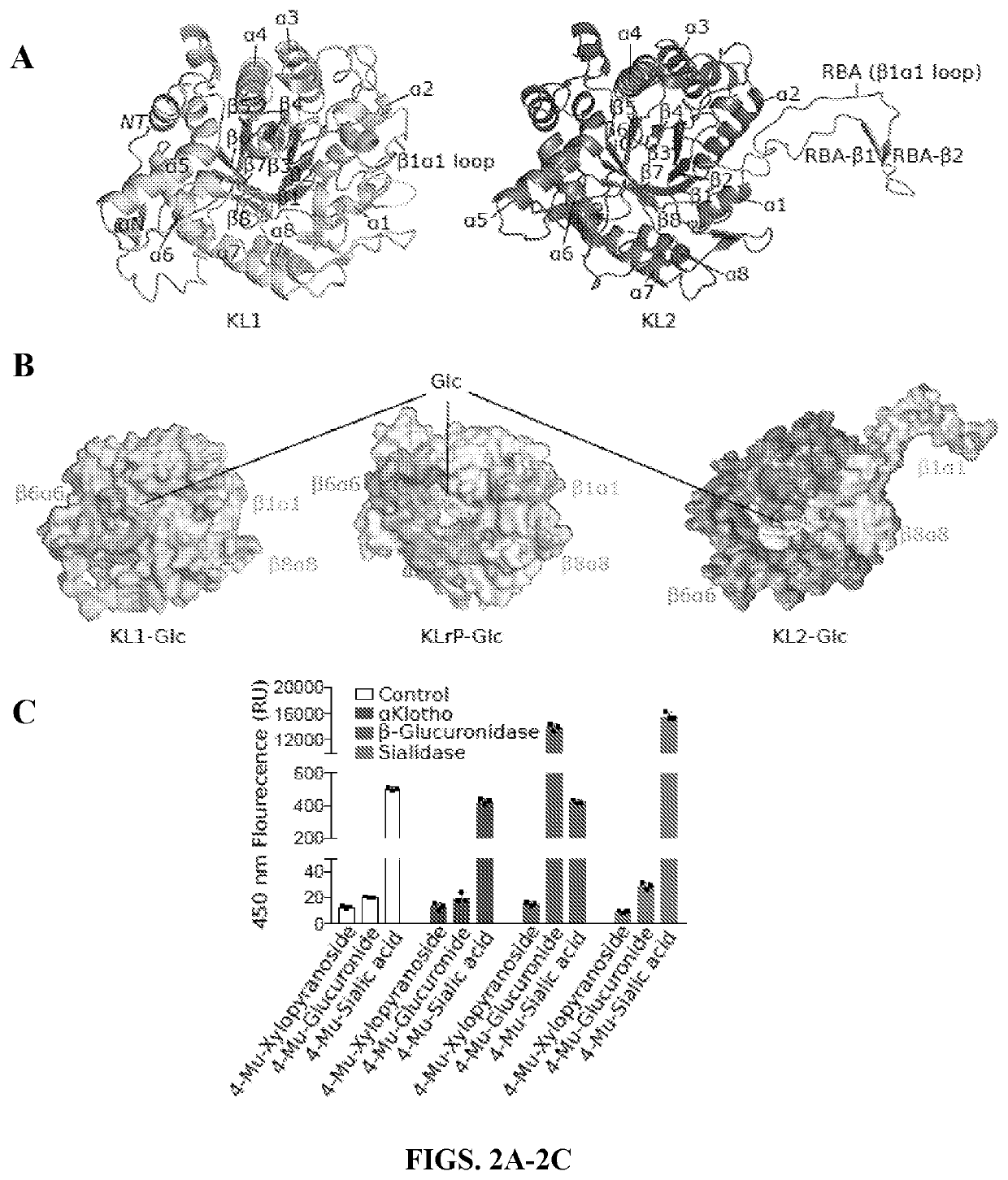
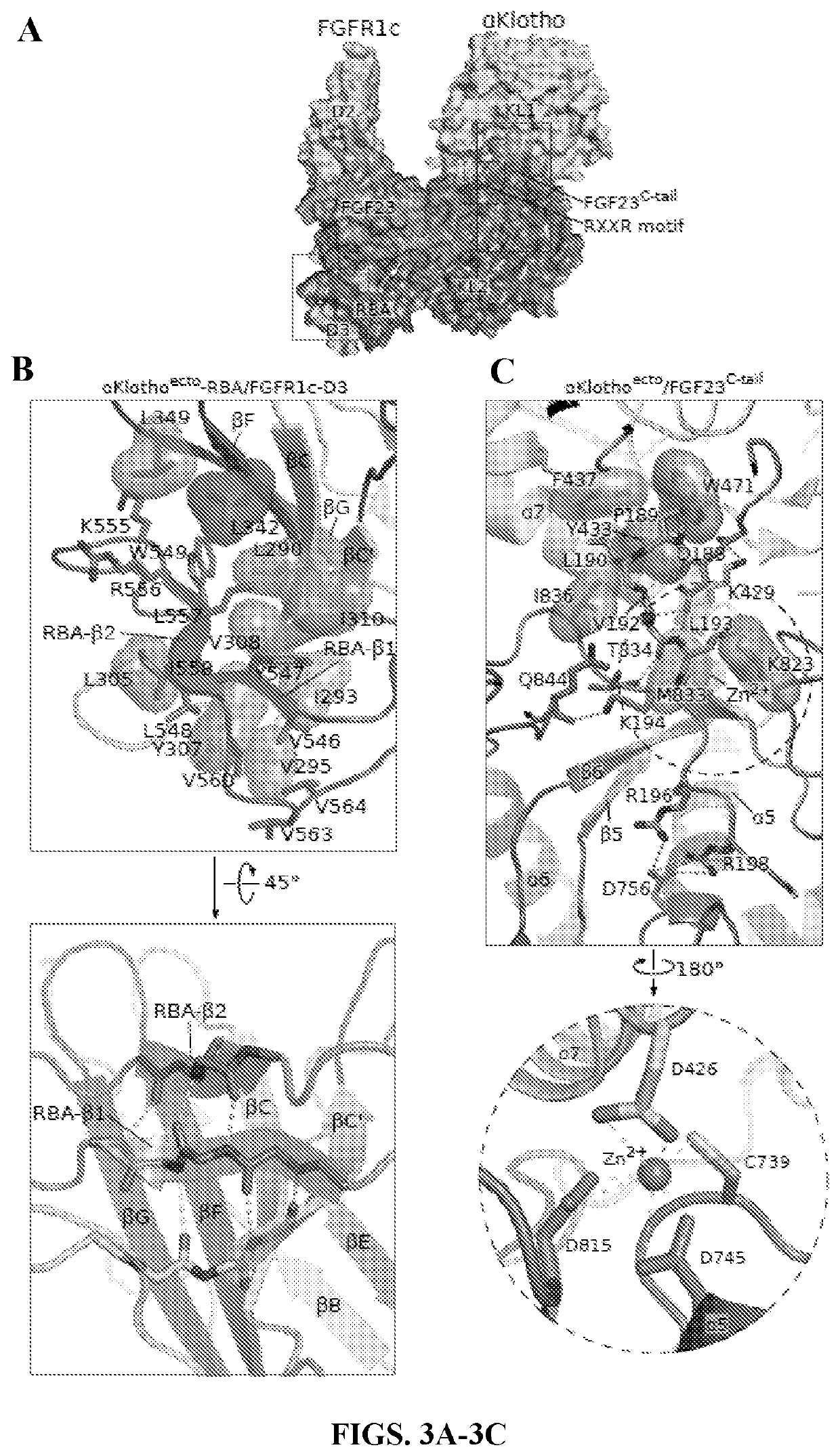
[0182]The interface between αKlotho RBA and FGFR1c D3 (FIG. 3A) buries over 2,200 Å2 of solvent-exposed surface area, which is consistent with the high affinity of αKlotho binding to FGFR1c (KD=72 nM)10. At the distal tip of the RBA, residues 547Tyr-Leu-Trp549 and 556Ile-Leu-Arg558 form a short β-strand pair (RBA-β1:RBA-β2) as their hydrophobic side chains are immersed in a wide hydrophobic groove between the four-stranded βC′-βC-βF-βG sheet and the βC-βC′ loop of FGFR1c D3 (FIG. 3B, upper panel). The RBA-β1:RBA-β2 strand pair forms an extended sheet with theβC′-βC-βF-βG sheet of D3 as the backbone atoms of RBA-β1 and D3 βC′ make three hydrogen bonds which further augment the interface (FIG. 3B, lower panel). Residues at the proximal end of the RBA engage a second smaller binding pocket at the bottom edge of D3 next to the hydrophobic groove (FIGS. 11A-11B). Both αKlotho binding pockets in the receptor D3 domain differ between “b” and “c” splice is...
example 4
nterface Between αKlotho and FGF23
[0184]Regions from both KL domains act together to recruit FGF23 (FIG. 1B), thus explaining why only an intact αKlotho ectodomain is capable of supporting FGF23 signaling (Kurosu et al., “Regulation of Fibroblast Growth Factor-23 Signaling by Klotho,”J. Biol. Chem. 281:6120-6123 (2006) and Wu et al., “C-Terminal Tail of FGF19 Determines its Specificity Toward Klotho Co-Receptors,”J. Biol. Chem. 283(48):33304-33309 (2008), each of which is hereby incorporated by reference in its entirety). The interactions between FGF23 and αKlotho result in the burial of a large amount of solvent-exposed surface area (2,732 Å2), of which nearly two-thirds (1961 Å2) are buried between the FGF23 C-terminal tail and αKlotho, with the remaining one-third buried between the FGF23 core and αKlotho (FIG. 3A). At the interface between αKlotho and FGF23 C-terminal tail, FGF23 residues 188Asp-Pro-Leu-Asn-Val-Leu193 adopt an unusual cage-like conformation (FIGS. 3A, 3C) which ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission energy | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com