Monoclonal antigen-binding proteins to intracellular oncogene products
a technology of monoclonal antibodies and oncogene products, applied in the field of chimeric antigen receptors, recombinant antibodies, etc., can solve the problems of difficult to make a drug selective for ras proteins, and the availability of traditional antibody-based strategies targeting cell surface antigens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Epitope Selection and Peptide Synthesis
[0078]For purposes of the present disclosure, K-Ras peptides are identified by amino acid position(s) relative to NCBI Reference Sequence NP_203524.1 of the NCBI protein database. Accordingly, the K-Ras peptides, LWVGAGGV and KLVVVGAGGV correspond to amino acids 6-14 and 5-14, respectively, of the reference sequence.
[0079]In order to select HLA-A2-restricted epitopes derived from Ras codon 12-mutations with strong immunogenicity, the prediction scores of Ras mutation sequences were first screened using three online available databases (BIMAS, RANKPEP and SYFPEITHI). (Ras mutation sequences are described in Smith et al. Oncogenic mutations in ras create HLA-A2.1 binding peptides but affect their extracellular antigen processing. International Immunology Vol. 9(8), pp. 1085-1093, 1997).
[0080]Based on the predicted binding scores from all three databases, the following peptides were selected for testing to determine if the peptides were able to el...
example 2
T2 Stabilization Assay
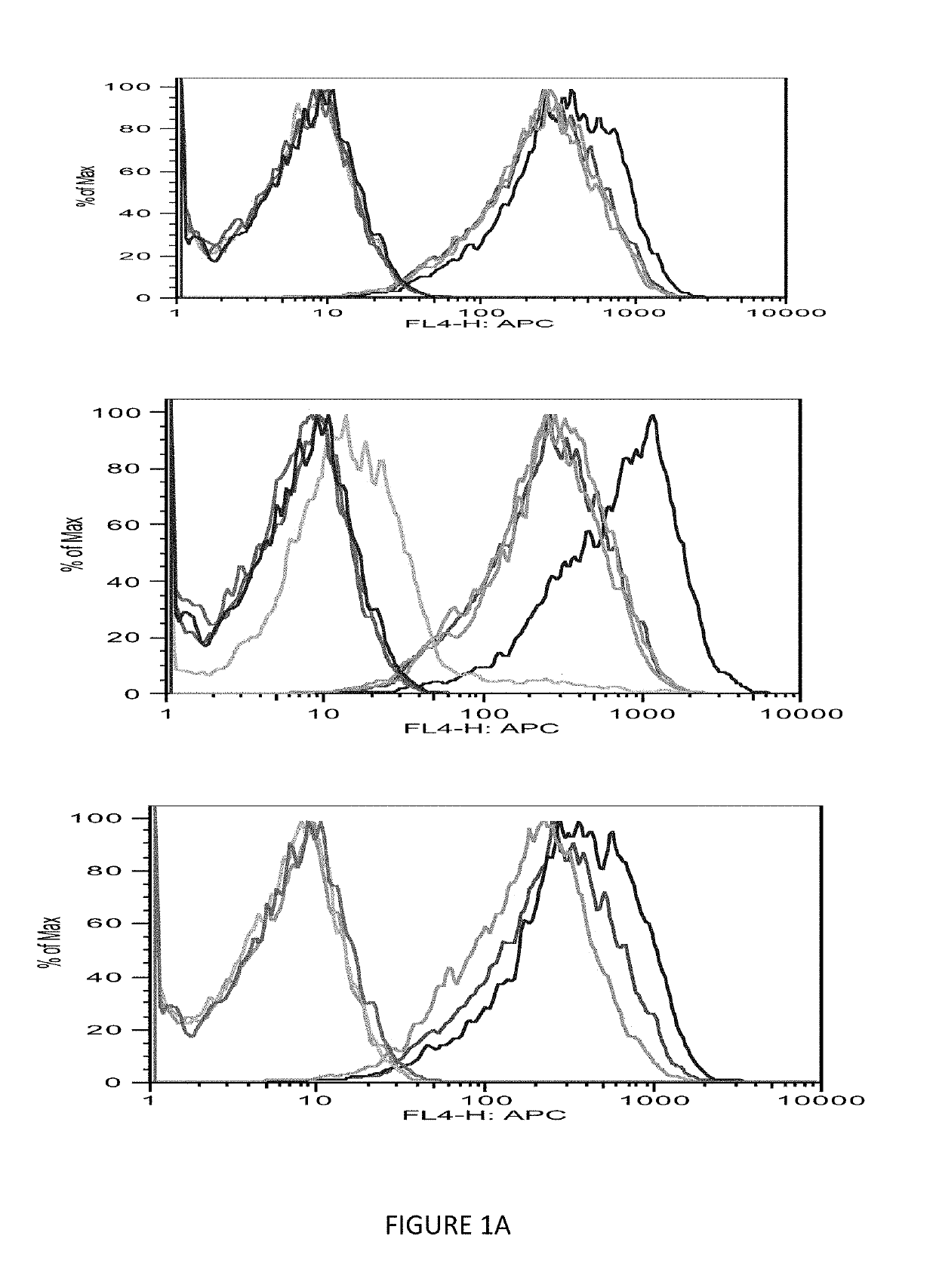
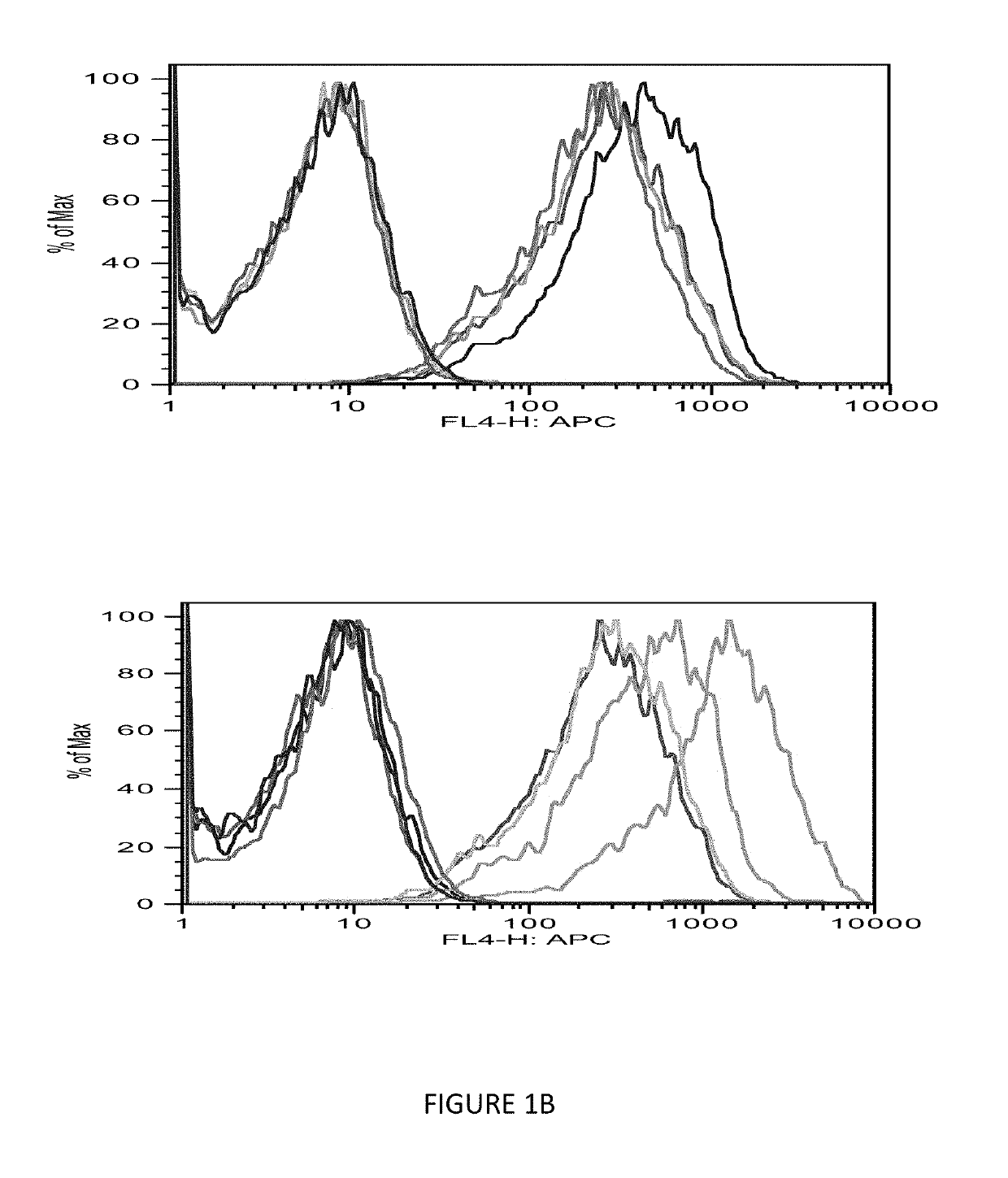
[0084]The immunogenicity of MHC class I-restricted peptides requires the capacity to bind and stabilize MHC class I molecules on the live cell surface. Moreover, the computer prediction has only up to 70% accuracy; so the next step was to seek a direct measurement of the strength of the interaction between the peptides and the HLA-A0201 molecules using a conventional binding and stabilization assay that uses antigen-transporting-deficient (TAP2 negative) HLA-A0201 human T2 cells. T2 cells lack TAP function and consequently are defective in properly loading class I molecules with antigenic peptides generated in the cytosol. The association of exogenously added peptides with thermolabile, empty HLA-A0201 molecules stabilizes them and results in an increase in the level of surface HLA-A0201 recognizable by a specific anti-HLA-A0201 mAb such as BB7.2.
[0085]The T2 binding assay showed that Ras 10-mer peptides wild type (WT), G12V, G12C, G12D increased the HLA-A2 exp...
example 3
Mutant Ras Peptide-Induced T Cell Responses
[0086]After informed consent on Memorial Sloan-Kettering Cancer Center Institutional Review Board approved protocols, peripheral blood mononuclear cells (PBMCs) from HLA-typed healthy donors were obtained by Ficoll density centrifugation. T cell stimulation followed the protocol described previously (Dao T. et al. Identification of a human cyclin D1-derived peptide that induces human cytotoxic CD4 cells. Plos One Vol. 4(9) e6730, 2009). Peptide-specific T cell responses were measured by IFN-g ELISPOT assay (Dao, T, Science Tr Med 2013).
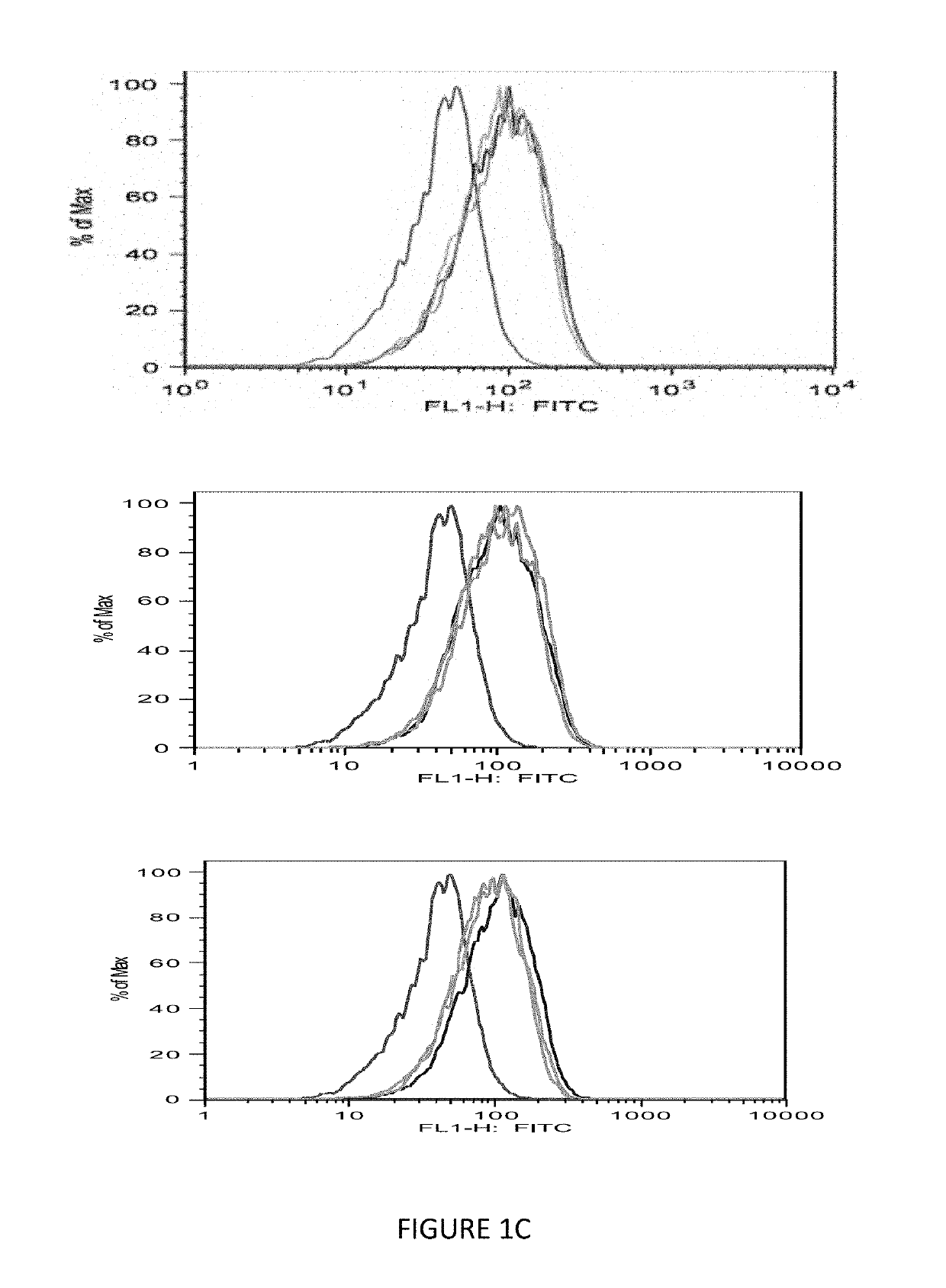
[0087]To expand the peptide-specific T cell precursors, three to five in vitro stimulations were performed and the specific T cell response was measured by IFN-g production, when challenged with individual peptide. Ras-G12V peptide stimulation induced strong T cell response against Ras10-G12V but showed no cross-reactivity to the peptides Ras10-WT, G12C and G12D (FIG. 2A). Similarly, Ras 9-G12V also induced s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
| nucleic acid | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com