Proteins for the treatment of epithelial barrier function disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Expression of SG-14
[0228]For experiments described in the examples below, a polynucleotide comprising a sequence encoding residues 1-631 of SEQ ID NO:3 (SG-14) was obtained by PCR amplification of genomic DNA obtained from Eubacterium eligens (C15-B4; DSM 3376 type strain; See, e.g., Holdeman, L. V., Moore, W. E. C. (1974) New genus, Coprococcus, twelve new species, and amended descriptions of four previously described species of bacteria from human feces. Int J Syst Bacteriol 24, 260-277. The encoding polynucleotide was then subcloned into an inducible expression vector and used to transform E. coli BL21(DE3) cells for expression and purification of SG-14 as detailed below, using culturing and purification methods which are routine in the art.
[0229]Expression and purification of proteins for use in various experiments pertaining to the present disclosure and for the following examples is described here. Expression was achieved using a pGEX vector system which is designed for induci...
example 2
Effect of SG-14 on Restoration of Epithelial Barrier Integrity Following Inflammation Induced Disruption
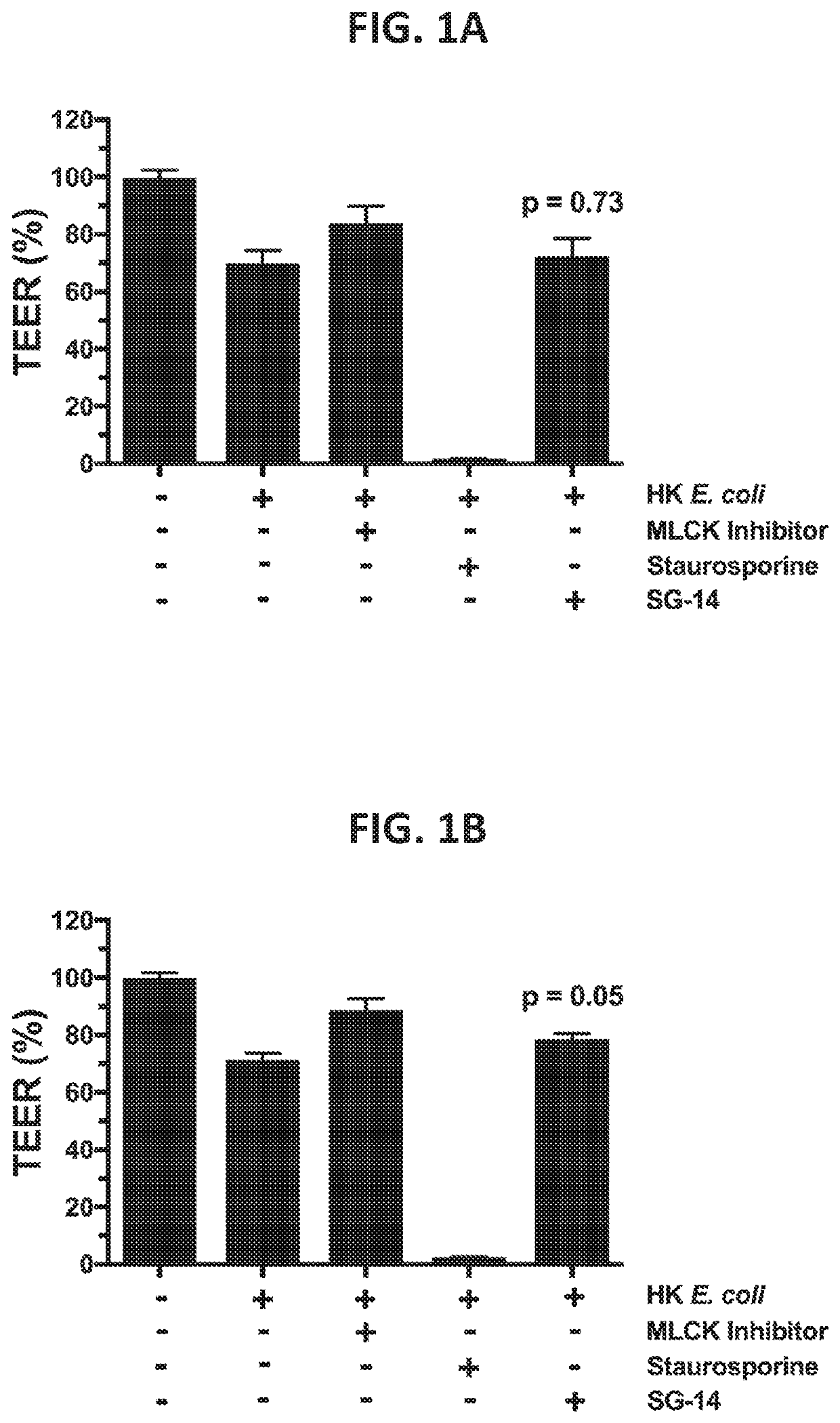
[0233]The following experiment demonstrates the therapeutic ability of a SG-14 protein or variant or fragment thereof as disclosed herein to restore gastrointestinal epithelial barrier integrity. The experiment is therefore a demonstration of the functional utility of the therapeutic protein SG-14 to treat a gastrointestinal inflammatory disorder or disease involving impaired epithelial barrier integrity / function.
[0234]Assays were performed as described below in trans-well plates where co-cultures of multiple cell types were performed utilizing a permeable membrane to separate cells. In the apical (top) chamber, human colonic epithelial cells, consisting of a mixture of enterocytes and goblet cells, were cultured until cells obtained tight junction formation and barrier function capacity as assessed by measurement of trans-epithelial electrical resistance (TEER). In the basolatera...
example 3
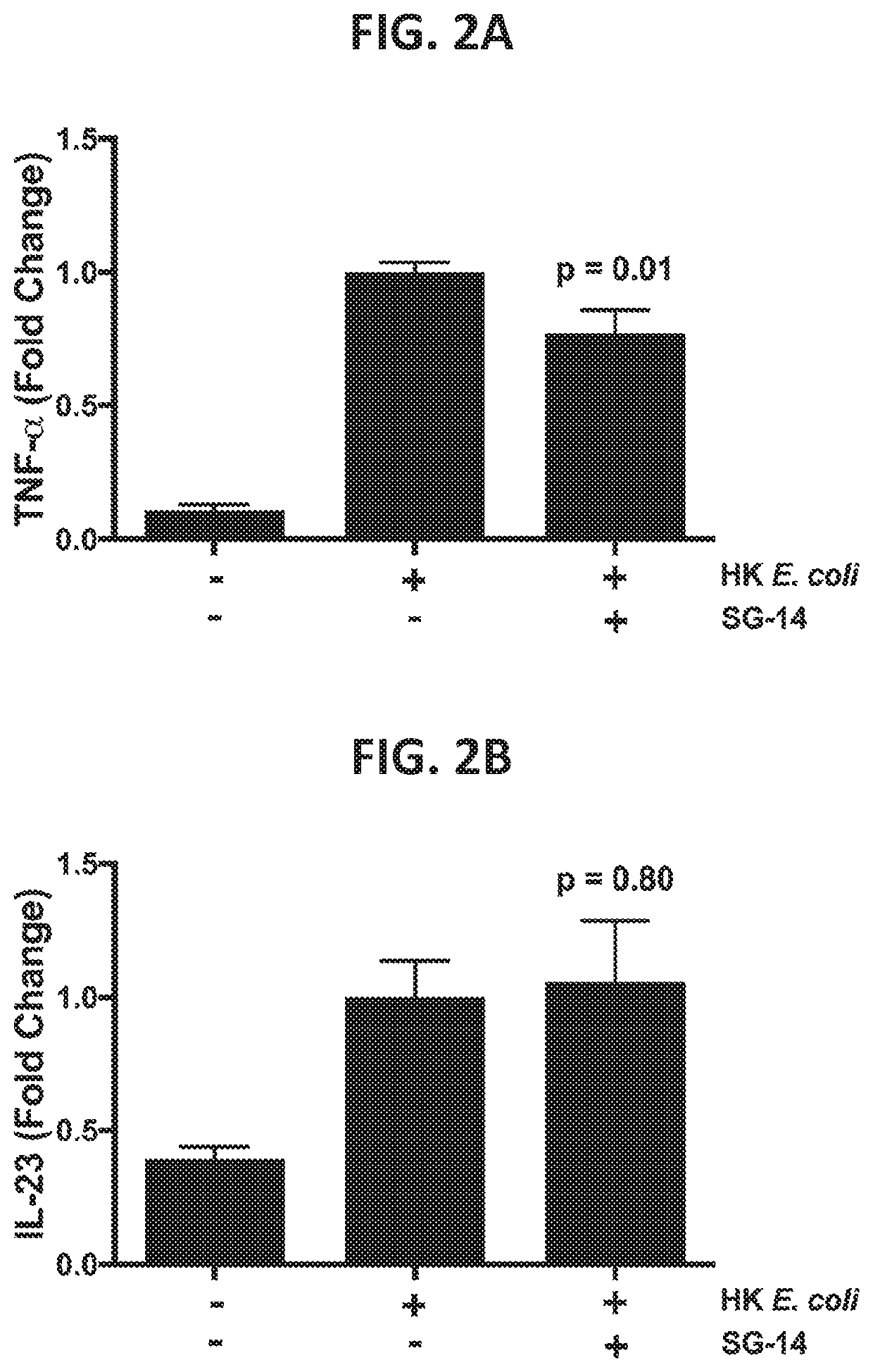
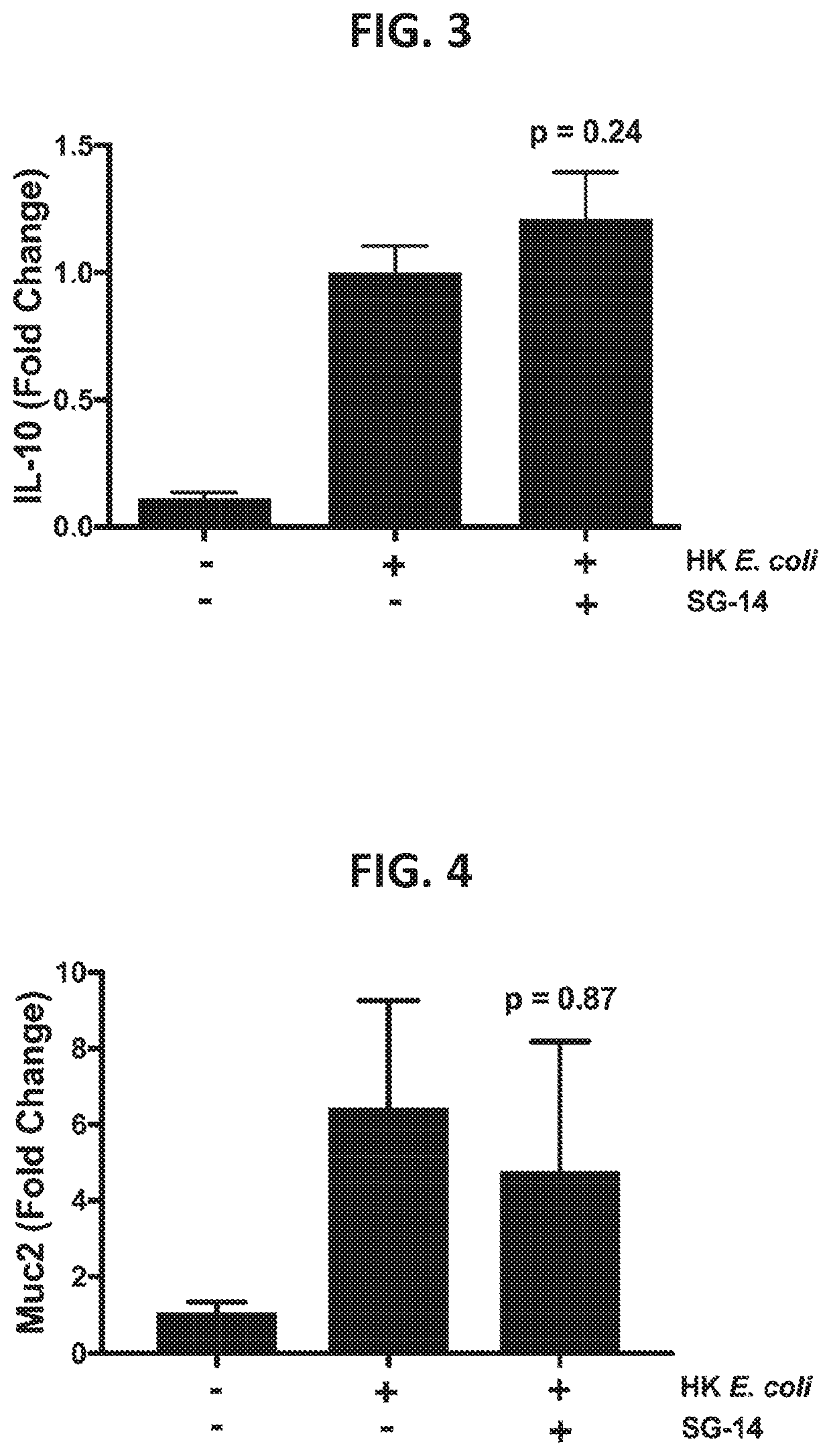
Effect of SG-14 on TNF-α and IL-23 Production in a TEER Assay
[0241]The following experiment demonstrates the therapeutic ability of a SG-14 protein or variant thereof as disclosed herein to reduce immune activation as measured by cytokine production. The experiment thereby demonstrates potential functional utility of the therapeutic protein to treat a gastrointestinal inflammatory disease, or disease involving impaired epithelial barrier integrity / function, where modulation of cytokine levels would affect the disease state in a host.
[0242]Production of the pro-inflammatory cytokines TNF-α and IL-23 by monocytes was measured in the tissue culture supernatant from the basolateral chamber of the co-culture TEER assay performed in Example 2. Following TEER readings, the supernatants were centrifuged at 10,000 g for 5 minutes at 4° C. to remove cell debris. Luminex analysis was performed according to the manufacturer's instructions (Magpix instrument and xPonent software version 4.2; Lum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com