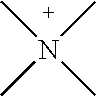
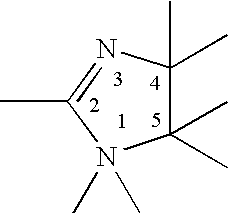
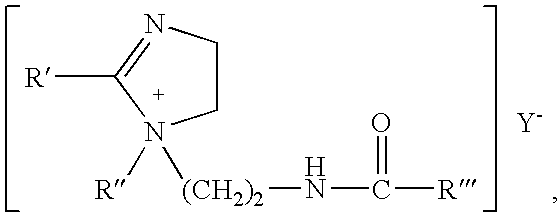
Mixtures of quaternary compounds
a technology of quaternary compounds and mixtures, applied in the field of chemical manufacture, can solve the problems of inherently unstable, high undesirable phase separation, and high utilization of emulsifiers, and achieve the effect of improving the formulation of hair conditioning products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 1-methyl-1-((erucylamido-)ethyl)-2-erucyl immidazolinium methyl sulfate
3132 g (4.62 moles) of erucic acid and 216 g (2.1 moles) of diethylenetriamine are placed in a dry stirred pressure vessel fitted with a nitrogen inlet. The vessel is purged with nitrogen and heated to 170° C. for 4-5 hours. The reaction mixture is then heated to 180° C. and vacuum is applied for another 4-5 hours. The reaction mixture is cooled to 95-100° C. and approximately 1.5 kg of cetearyl alcohol is added. The reaction mixture is further cooled to 75-80° C. and 250 g of dimethyl sulfate is slowly added with stirring. Once all dimethyl sulfate is added, the reaction mixture is held at 75-80° C. for approximately one hour, providing 1-methyl-1-((erucylamido-)ethyl)-2-erucyl immidazolinium methyl sulfate as the product.
example 2
Preparation of 1-methyl-1-(erucic rapeseed-)-ethyl)-2-(erucic rapeseed-)immidazolinium methyl sulfate (Mixture of dialkyl imidazoline Quats of Hydrogenated Rapeseed Oil)
1843.6 g (1.88 moles) of hydrogenated rapeseed oil and 283.34 g (2.75 moles) of diethylenetriamine were placed in a dry stirred pressure vessel fitted with a nitrogen inlet. The vessel was purged with nitrogen and heated to 165° C. for 5 hours until a base value of 76 was reached. The reaction mixture was then heated to 190° C. and vacuum was applied for 5 hours to obtain a 94% tertiary amine content. The resulting imidazoline intermediate was then cooled to 95° C. and 1772 g of cetearyl alcohol were added to act as solvent. The reaction mixture was further cooled to 85° C. and 330 g (2.6 moles) of dimethyl sulfate were slowly added over a 30-minute period with stirring. Once all dimethyl sulfate was added, the reaction mixture was held at 85-90° C. for another 60 minutes. The resulting light yellow solid product inc...
example 3
Preparation of 1-methyl-1-N-(n-propyl)-2-erucyl immidazolinium methyl sulfate
% of totalTotalcationiccationicComponentIngredientactivityactivityComponent (a)Mixture of70%45%Table 1Component (b)Compound30%(XXIII)SolventMixture of—cetearylalcohol (80%)and 1,3-butanediol(20%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com