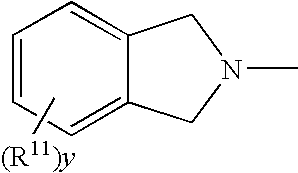
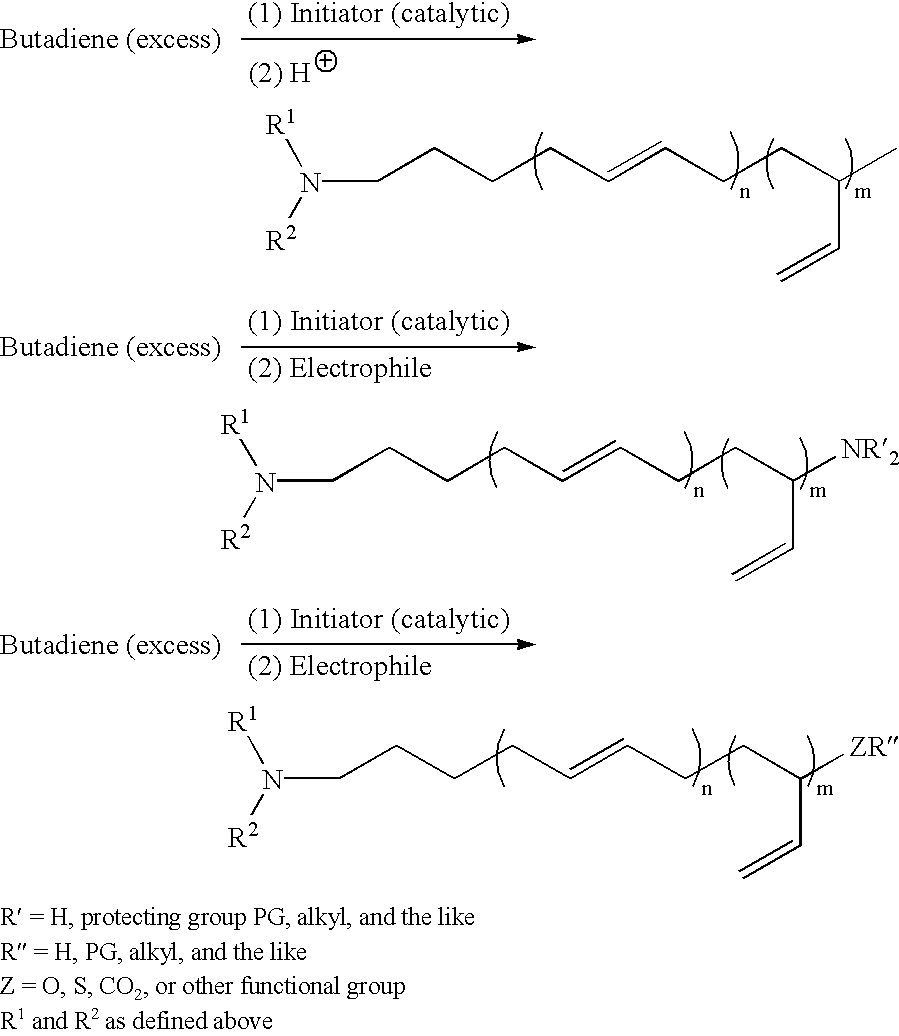
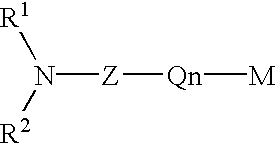Protected aminofunctionalized polymerization initiators and methods of making and using same
a polymerization initiator and functional technology, applied in the field of polymerization initiators, can solve the problems of reducing the amount of 1,2-microstructure in the resultant polymer, etc., and achieving the effect of commercial production of functionalized polymers at 78° c. and thf solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Initiator Precursor 3-[(N-benzyl-N-methyl)amino]-1-propylchloride
[0135]To a stirred suspension of K2CO3 (200 g, 1.5 mole) in cyclohexane 200 mL and 1-bromo-3-chloropropane (“BCP”) (540 g, 3.4 mole) was added dropwise over a period of 1 hour at 20° C. benzylmethyl amine (272 g, 2.24 mole). After complete addition the reaction was allowed to stir for an additional 20 hours. The crude reaction mixture was filtered and then washed with saturated NaCl (3×100 mL). The organic phase was extracted with 3N HCl (3×100 mL). The resulting aqueous phase, containing the desired product as the hydrochloric salt, was washed with hexanes (3×100 mL) to remove any residual BCP. The aqueous phase was subsequently basified with 50 wt % NaOH and extracted with cyclohexane (3×100 mL). After solvent removal 220 g (50% yield) of the title compound was isolated as a yellow oil.
example 2
Preparation of 3-[(N-benzyl-N-methyl)amino]-1-propyllithium
[0136]To a 500 ml Morton / cleave flask reactor under argon atmosphere was added lithium powder (13.32 g, 1.92 mole) and 141 grams of cyclohexane. To a constant addition funnel was added 3-[(N-benzyl-N-methyl)amino]-1-propylchloride (70.93 g, 0.34 mol) and 83.3 grams cyclohexane. Immediately before beginning the addition, the lithium metal mixture was heated to 53° C. using a heating mantel. Dropwise addition of the feed solution was performed while maintaining the reaction temperature at 50° C. A cooling bath of hexane, to which dry ice was added periodically, was employed to maintain a reaction temperature between 48° to 51° C. The total addition time was 1.22 hours with an average stirring rpm of 925. The reaction mixture was stirred at least one hour after the feed was completed. This mixture was then pumped through a ⅜″ teflon tube to a pressure filter that contained about 10 grams of filter aid and filtered under an argo...
example 3
Preparation of Initiator Precursor 3-[(N,N-dibenzylamino]-1-propylchloride
[0137]To a stirred suspension of K2CO3 (200 g, 1.5 mole) in cyclohexane 200 mL and 1-bromo-3-chloropropane (540 gms, 3.4 mole) is added dropwise over a period of 1 h at 20° C. dibenzyl amine (442 g, 2.24 mole). After complete addition the reaction is allowed to stir for an additional 20 h. The crude reaction mixture is filtered and then washed with saturated NaCl (3×100 mL). The organic phase is extracted with 3N HCl (3×100 mL). The resulting aqueous phase, containing the desired product as the hydrochloric salt, is washed with hexanes (3×100 mL) to remove any residual BCP. The aqueous phase is subsequently basified with 50 wt % NaOH and extracted with cyclohexane (3×100 mL). After solvent removal 324 g (53% yield) of the title compound was isolated as a yellow oil.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com