Anti-C5 antibodies
a technology of anti-c5 antibodies and antibodies, applied in the field of anti-c5 antibodies, can solve the problems of marked impairment of the plasma retention of igg, and achieve the effect of enhancing the clearance of c5
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of C5
1.1. Expression and Purification of Recombinant Human and Cynomolgus Monkey C5
[0354]Recombinant human C5 (NCBI GenBank accession number. NP_001726.2, SEQ ID NO: 39) was expressed transiently using FreeStyle293-F cell line (Thermo Fisher, Carlsbad, Calif., USA). Conditioned media expressing human C5 was diluted with equal volume of milliQ water, then applied to a Q-sepharose FF or Q-sepharose HP anion exchange column (GE healthcare, Uppsala, Sweden), followed by elution with a NaCl gradient. Fractions containing human C5 were pooled, then salt concentration and pH was adjusted to 80 mM NaCl and pH6.4, respectively. The resulting sample was applied to a SP-sepharose HP cation exchange column (GE healthcare, Uppsala, Sweden) and eluted with a NaCl gradient. Fractions containing human C5 were pooled and subjected to CHT ceramic Hydroxyapatite column (Bio-Rad Laboratories, Hercules, Calif., USA). Human C5 eluate was then applied to a Superdex 200 gel filtration column (G...
example 2
Generation of Anti-C5 Antibodies
2.1. Antibody Screening
[0357]Anti-C5 antibodies were prepared, selected and assayed as follows:
[0358]Twelve to sixteen week old NZW rabbits were immunized intradermally with human C5 and / or monkey C5 (50-100 μg / dose / rabbit). This dose was repeated 4-5 times over a 2 month period. One week after the final immunization, the spleen and blood were collected from the immunized rabbits. Antigen-specific B-cells were stained with labelled antigen, sorted with FCM cell sorter (FACS aria III, BD), and plated in 96-well plates at one cell / well density together with 25,000 cells / well of EL4 cells (European Collection of Cell Cultures) and activated rabbit T-cell conditioned medium diluted 20 times, and were cultured for 7-12 days. EL4 cells were treated with mitomycin C (Sigma, Cat No. M4287) for 2 hours and washed 3 times in advance. The activated rabbit T-cell conditioned medium was prepared by culturing rabbit thymocytes in RPMI-1640 containing Phytohemagglut...
example 3
Binding Characterization of Anti-C5 Antibodies
3.1. Expression and Purification of Recombinant Antibodies
[0369]Recombinant antibodies were expressed transiently using FreeStyle293-F cell line (Thermo Fisher, Carlsbad, Calif., USA). Purification from the conditioned media expressing antibodies was performed using a conventional method using protein A. Gel filtration was further conducted if needed.
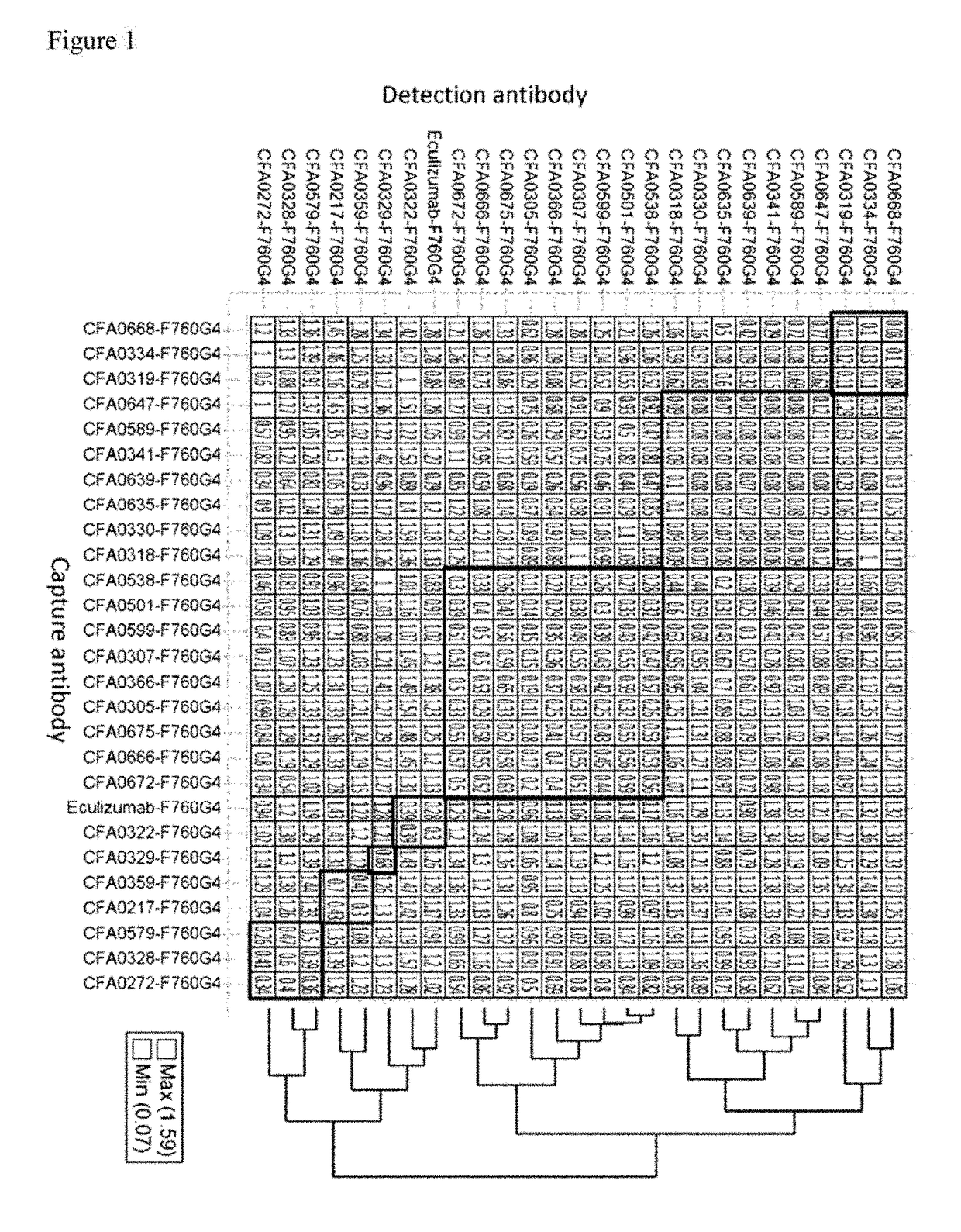
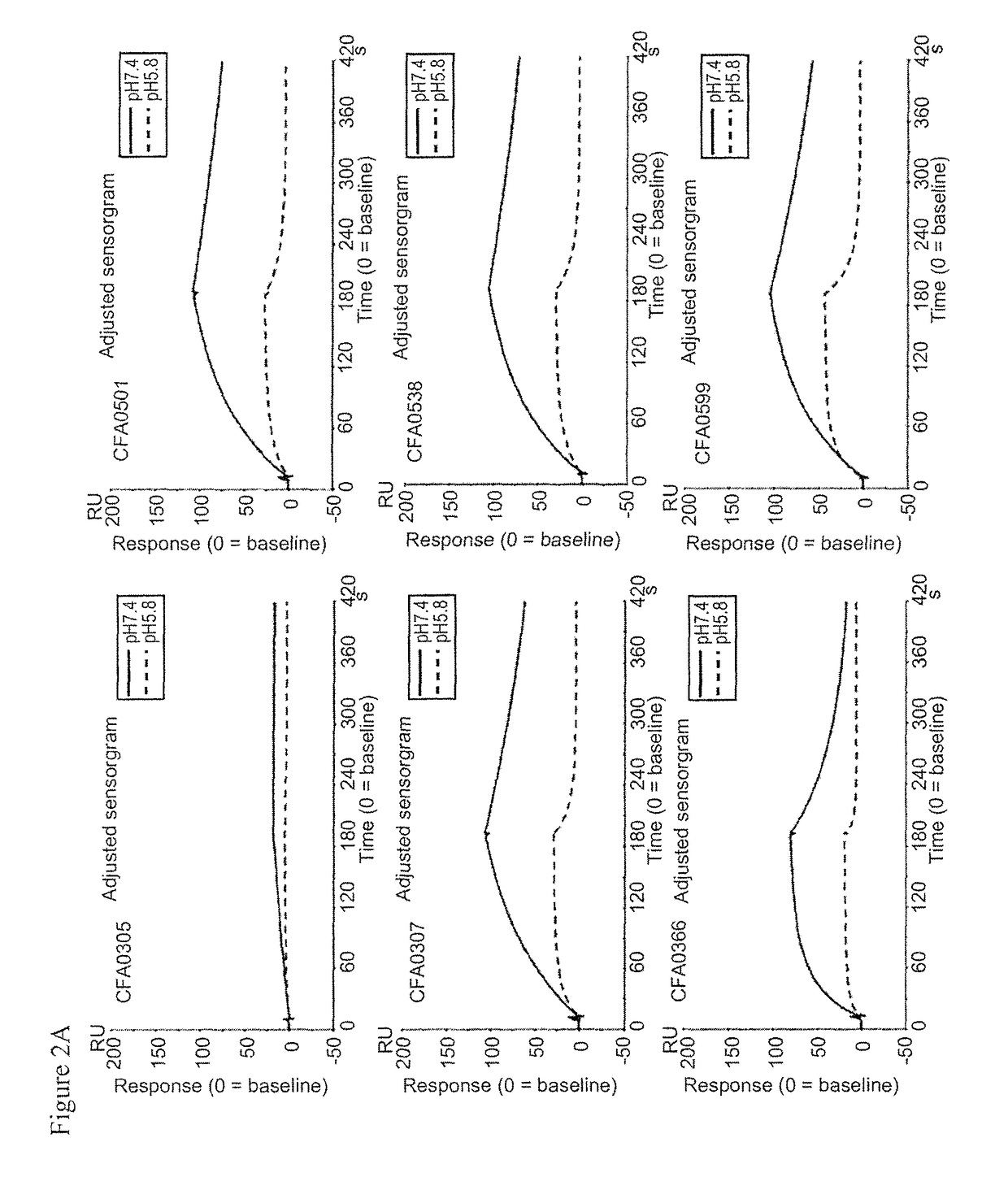
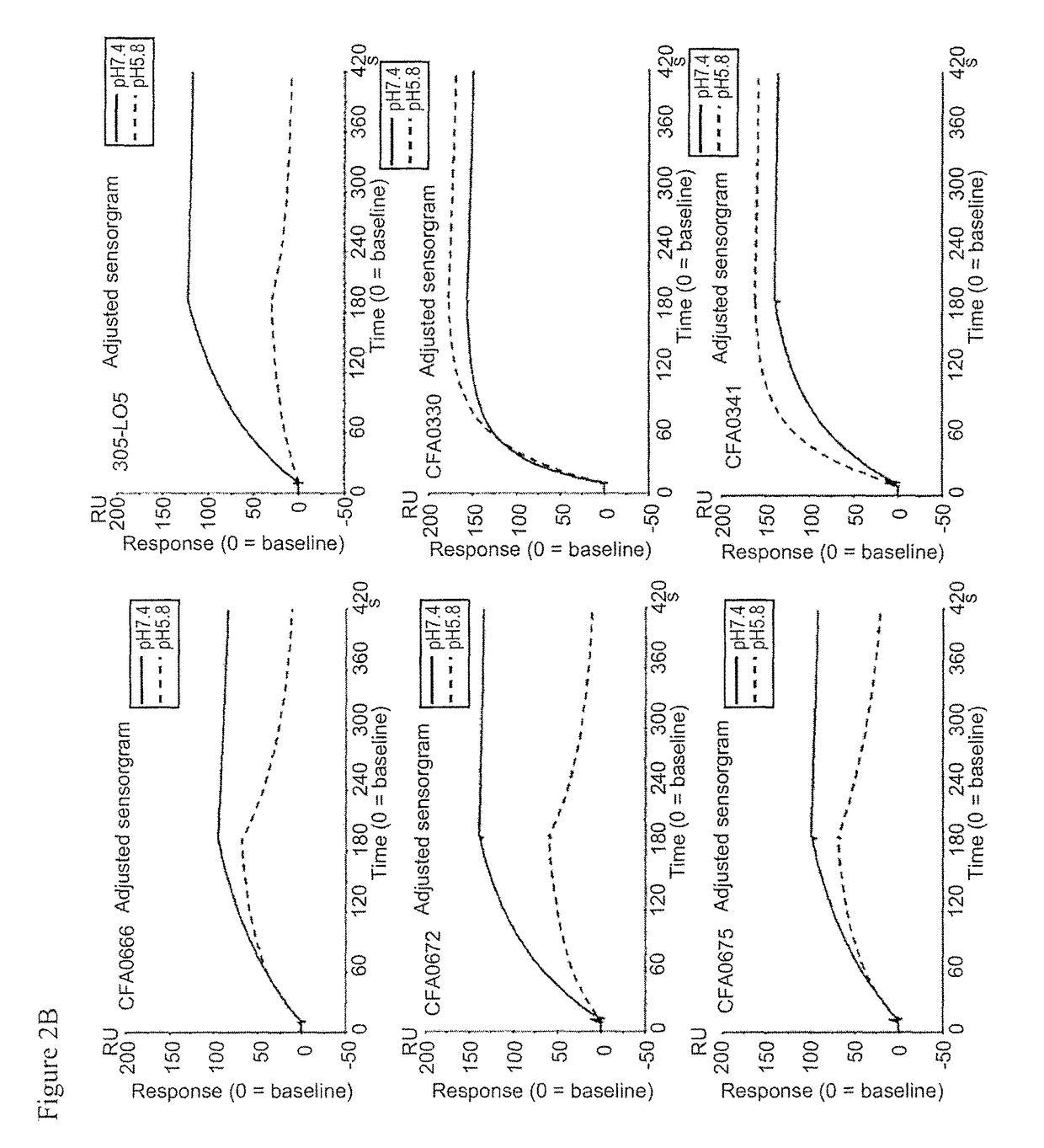
3.2. Assessment of pH Dependency
[0370]The kinetic parameters of anti-C5 antibodies against recombinant human C5 were assessed at pH7.4 and pH5.8, at 37° C. using BIACORE® T200 instrument (GE Healthcare). ProA / G (Pierce) was immobilized onto a CM4 sensorchip using amine coupling kit (GE Healthcare) according to the recommended settings by GE Healthcare. Antibodies and analytes were diluted into the respective running buffers, ACES pH7.4 and pH5.8 (20 mM ACES, 150 mM NaCl, 1.2 mM CaCl2, 0.05% Tween 20, 0.005% NaN3). Each antibody was captured onto the sensor surface by ProA / G. Antibody capture...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com