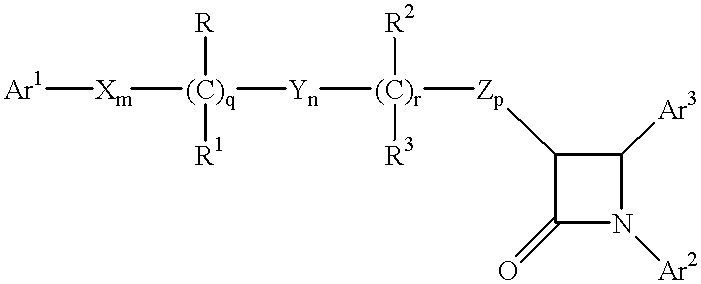
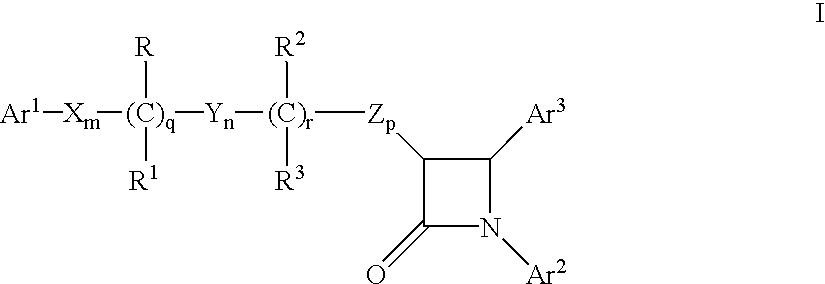
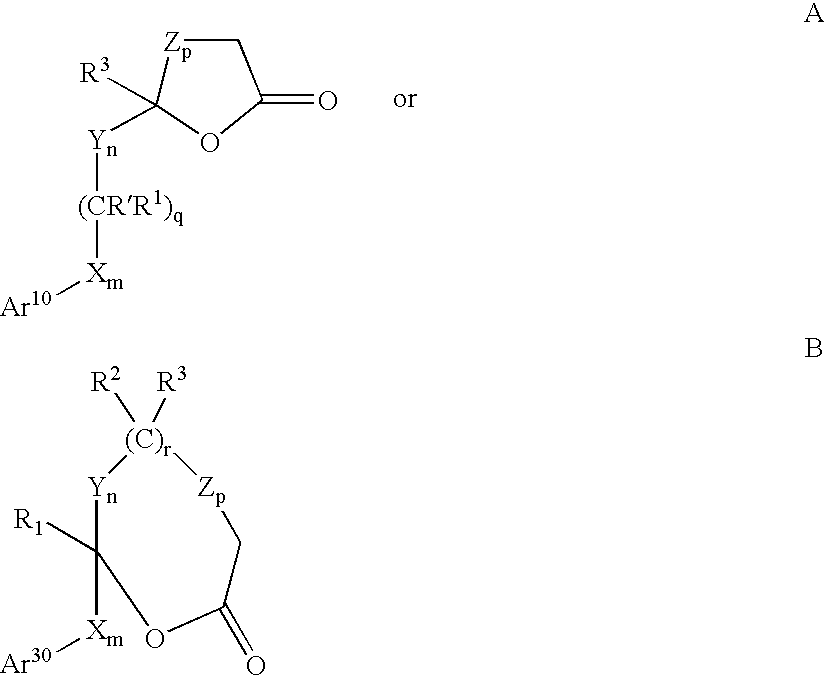
Hydroxy-substituted azetidinone compounds useful as hypocholesterolemic agents
a technology of azetidinone and hydroxysubstituted azetidinone, which is applied in the field of hydroxysubstituted azetidinone, can solve the problems of significant elevation of the risk of chd, and achieve the effects of reducing plasma cholesterol, preventing atherosclerosis, and lowering serum cholesterol levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
##STR30##
To a solution of compound A from Example 1 (0.5 g, 1.3 mmol) in anhydrous pyridine (2.7 ml), add acetic anhydride (0.63 ml, 6.7 mmol). Stir for 16 h, dilute with CH.sub.2 Cl.sub.2 and wash 3.times. with 1N HCl 1.times. with NaCI (sat'd) and 1.times. with water. Concentrate the organic layer to dryness and crystallize the residue from EtOAc to obtain the title compound (0.46 g), mp 167.degree.-169.degree. C.; IR 1745 cm-1; EI (M+) 415; J=5.9 Hz.
example 3
##STR31##
Freshly prepare a solution of lithium isopropylcyclohexylamide (LICA) by adding n-butyllithium (2.84 mL of a 1.6M solution) to 5 a solution of isopropylcyclohexylamine (0.75 mL) in THF (100 mL) at -78.degree. C. Dissolve N-phenyl-4-(4-methoxyphenyl)-2-azetidinone (1.0 g) in THF (8 mL) and slowly add to the LICA solution at -78.degree. C. After stirring for 20 min, add hydrocinnamaldehyde (0.54 g) and stir the reaction mixture at -78.degree. C. for 4 h. Quench the reaction with 10% KHSO.sub.4 and extract the product with EtOAc. Separate the organic layer, wash with water and NaCl (sat'd). Concentrate the extract and purify the resultant residue on a silica gel 60 column, eluting with EtOAc:hexane (15:85) to obtain 1.15 g of product as a mixture of diastereomers. Separate the diastereomers by HPLC on a silica gel column to give three diastereomers 3A, 3B and 3C:
3A ##STR32## 1H in CDCl.sub.3 :7.32-7.18(m, 11H); 7.08-6.99 (m, 1H); 6.89(d, J = 9 Hz, 2H); 4.80(d, J = 2.4 Hz, 1H);...
example 4
##STR40##
Method 1:
Step 1) To a refluxing solution of of 4-methoxyberizylidene anisidine (10.0 g, 41.5 mmol) and tributylamine (20.8 ml, 87 mmol) in toluene (100 ml), add 5-bromovaleroyl chloride (8.5 g, 43, mmol) in toluene (20 ml) dropwise over 2 h. Stir the reaction mixture at 80.degree. C. for 12 h, cool to room temperature, wash 3.times. with 1 N HCl, 1.times. with water and dry the organic layer over MgSO.sub.4. Purify by silica gel chromatography, eluting with ethyl acetate:hexane (4:1) to obtain 5.1 g of (3R, 4S)-1,4-bis(4-methoxyphenyl)-3-(3-bromoproyl)-2-azetidinone (relative stereochemistry), mp 70.degree.-73.degree. C., El (M.sup.+) 404; J=2.3 Hz.
Step 2) To a solution of the product of step 1 (5.1 g, 12.6 mmol) in (CH.sub.3).sub.2 SO (20 ml), add (CH.sub.3).sub.3 N(O) (2.39 g, 31.9 mmol). Heat the mixture at 60.degree. C. for 3 h, cool to room temperature, dilute with EtOAc, and wash 3.times. with water. Combine the aqueous fractions and extract with EtOAc. Combine the or...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com