Compositions and methods for modulating serum cholesterol
a technology of serum cholesterol and compositions, applied in the direction of peptide/protein ingredients, metabolism disorders, instruments, etc., can solve the problems of incomplete characterization of tnf- receptors, significant side effects, and cholesterol uptake, so as to enhance the activity of ldl receptors, enhance the selectivity and effectiveness of testing, and enhance the production of ldl receptors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Effect of TNF-α on Neutral Sphingomyelinase Activity
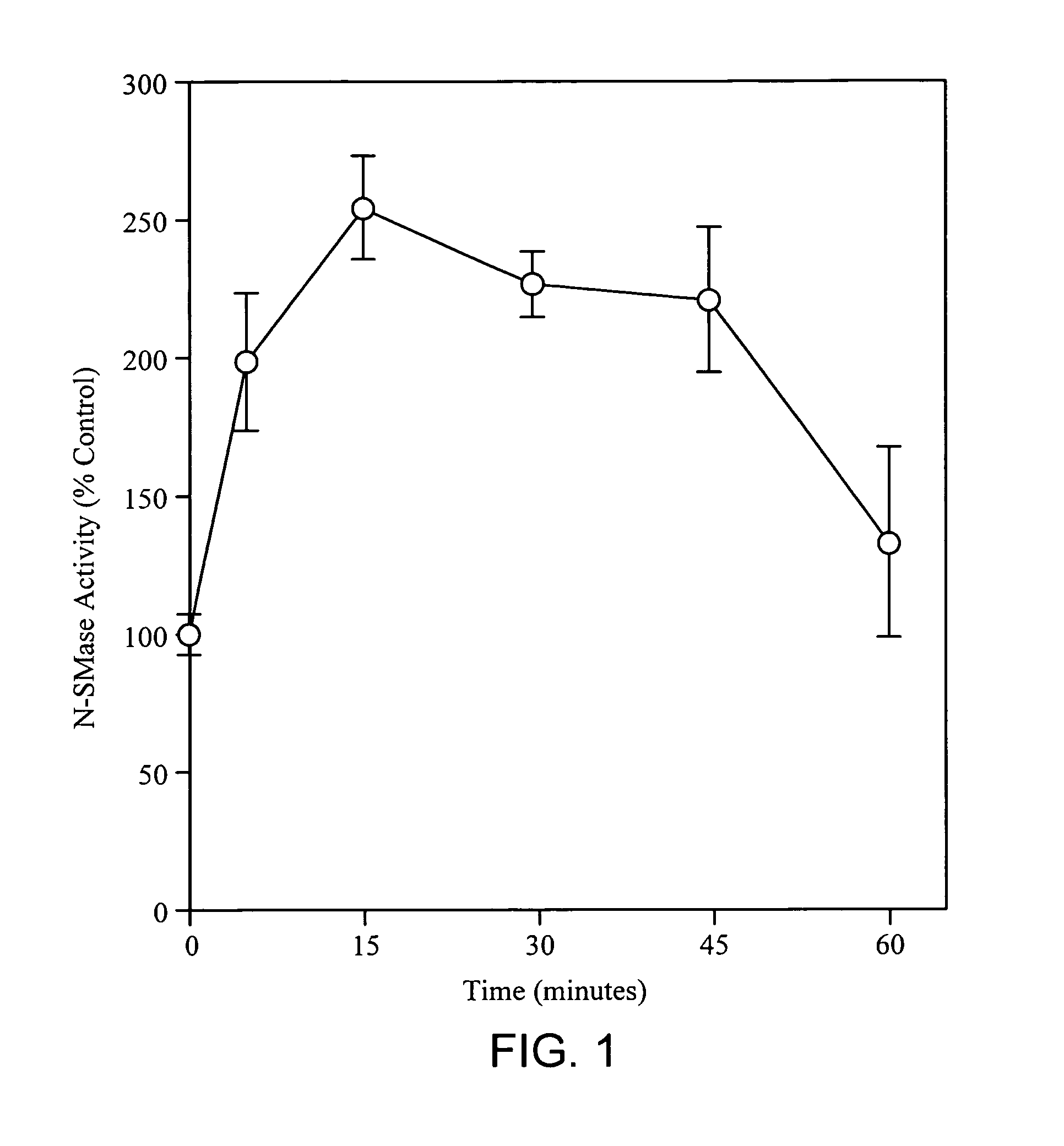
[0146] Neutral sphingomyelinase activity increased rapidly with the addition of TNF-a. See FIG. 1. A maximal 2.5 fold increase in activity was observed 15 minutes after TNF-α was added to the cells. The gradual return of N-SMase activity to control levels within 1 hour contrasted the rapid onset of activation and is reflected in the asymmetric kinetic profile observed.
[0147]FIG. 1 illustrates the effect of TNF-α on neutral sphingomyelinase activity and is explained in more detail as follows: Confluent cultures of HH-25 cells were washed once with PBS and incubated in serum free media for 30 minutes prior to the addition of TNF-α (10 ng / ml). At the indicated time, cells were harvested in PBS, pelleted and frozen. Cells were subsequently lysed as described in materials and methods. N-SMase assays were performed in duplicate as described. Error bars represent±one standard deviation from the mean.
example 2
Kinetics of SREBP-1 Proteolysis
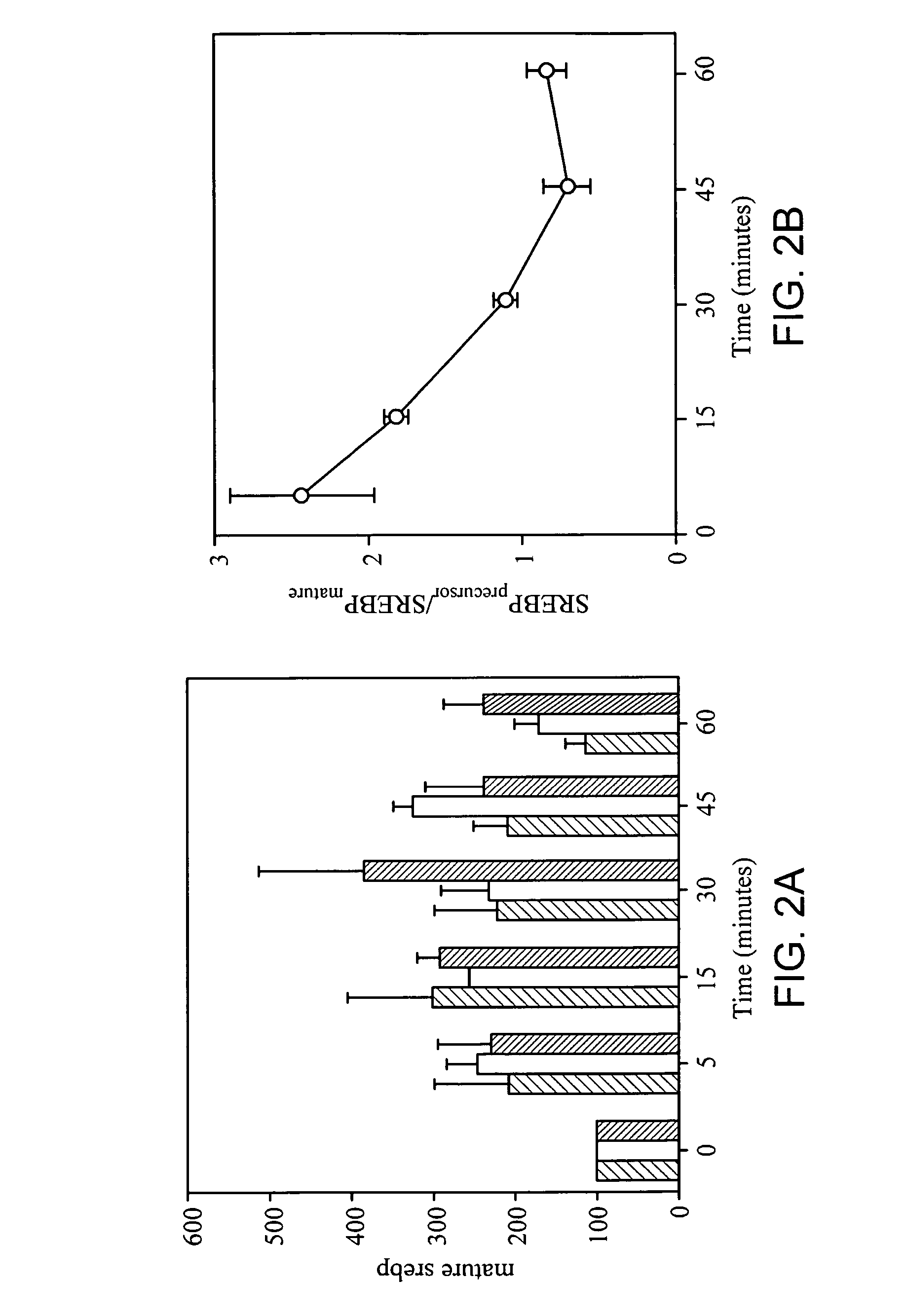
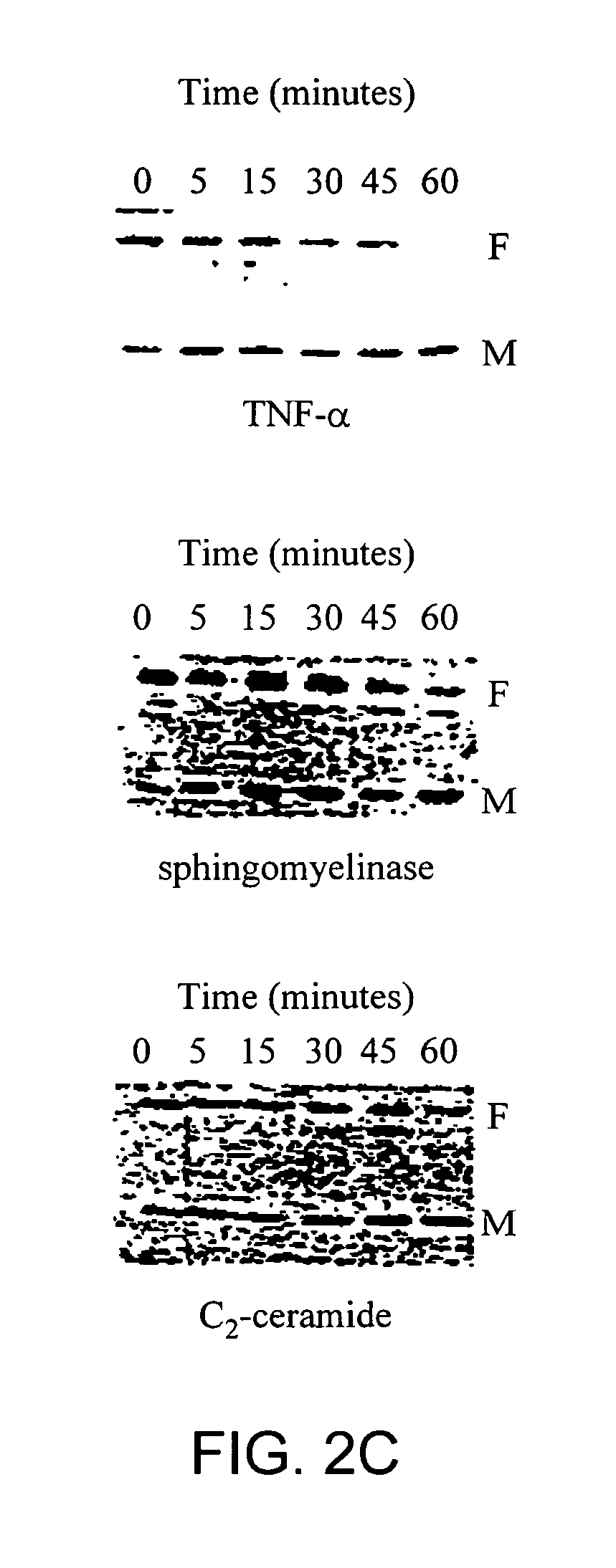
[0148] Sterol independent SREBP-1 maturation in response to TNF-α closely paralleled the kinetics of TNF-α induced N-SMase activation. The mass of the mature form of SREBP-1 was found to increase 2 fold after 5 minutes and 3 fold after 15 minutes of incubation with TNF-α. See FIG. 2A. The amount of mature SREBP-1 returned to control levels within one hour. This effect could not be recapitulated with EGF or PDGF treatment. The increase in mature SREBP-1 levels was accompanied by a concomitant decrease in the intensity of the band corresponding to the precursor form of SREBP-1. See FIG. 2B. After 60 minutes of treatment significantly less precursor SREBP-1 was visible.
[0149] To incorporate the observed increase in mature SREBP-1 and the concomitant decrease in precursor SREBP-1 into a single variable, the ratio of precursor SREBP-1 to mature was plotted. See FIG. 2B. A maximal 1.5 fold decrease in the precursor to mature ratio occurred 45 minutes after...
example 3
Effects of TNF-α, Sphingomyelinase and C2-ceramide on Apoptosis in Hepatocytes
[0155] To demonstrate that the observed maturation of SREBP-1 was not an artifact of the more general phenomenon of apoptosis induced proteolysis we performed DNA laddering assays. The 160 bp DNA ladder characteristic of cells undergoing apoptosis was not observed in any of the samples.
[0156] TNF-α, C2-ceramide and sphingomyelinase did not induce apoptosis demonstrating that in hepatocytes, SREBP-1 maturation is not part of the more general phenomenon of apoptotic protein hydrolysis.
PUM
| Property | Measurement | Unit |
|---|---|---|
| w/w | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com