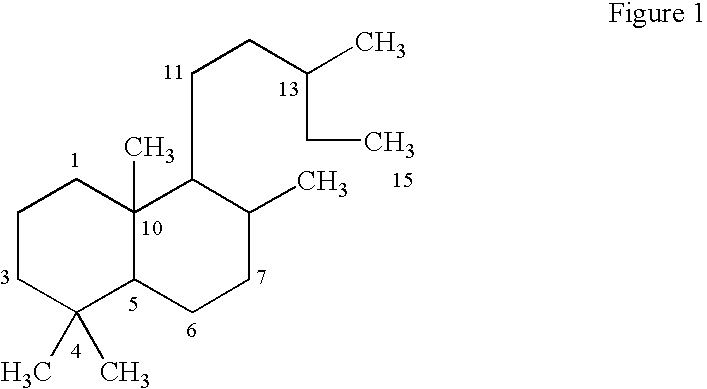
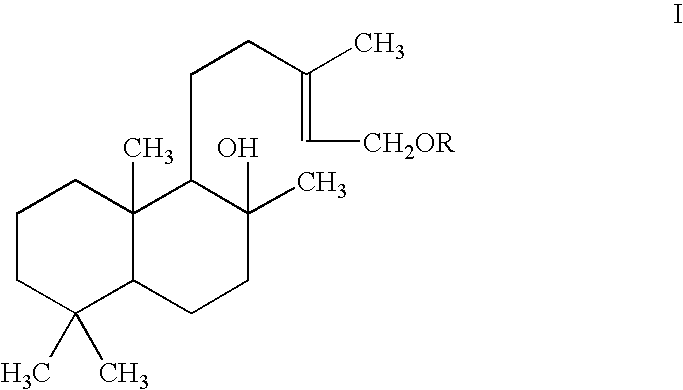
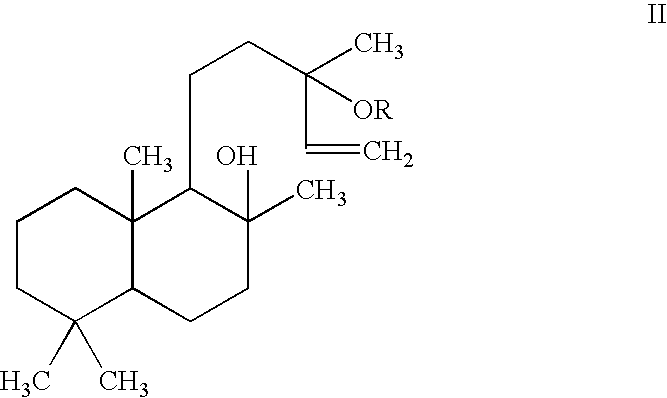
Pharmaceutical formulations comprising labdanes for the treatment of tumors or leukemias
a technology for cancer and labdane, which is applied in the direction of biocide, plant/algae/fungi/lichens, drug compositions, etc., can solve the problems of unstable encapsulation, inefficient encapsulation, and early results that are disappointing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Labd-13-end,8α-ol,15-yl Acetate
[0052]Labd-13-end,8α,15 diol (I) (50 mg) was dissolved in 2 ml of Ac2O-Py (acetic anhydrate-pyridine) for 48 hours at room temperature. The reaction mixture evaporated in vacuum to remove the solvents The purity as well the identification of the compound labd-13-ene,8α-ol,15-yl acetate was tested by TLC (Thin Layer Chromatography) and GC-MS (Gas Chromatography-Mass Spectrometry), using chromatography data. Compound was obtained in its pure state (47 mg).
example 2
Labd-13-ene-8α-ol 15-yl-β(or -α)-D (or -L)-pyrano (or furano)sides as Monosaccharides or as Disaccharides
[0053]Labd-14-ene-8α-ol 13-yl-β(or -a)-D (or -L)-pyrano (or furano)sides as monosaccharides or as disaccharides. 3-yl-β(or -a)-D (or -L)-pyrano (or furano)sides as monosaccharides or as disaccharides, Labd-14-ene, 8, 13-epoxy As an example
[0054]Condensation of Labd-13-ene-8α,15-diol (I) with 2,3,4,6-tetra-O-acetyl-a-D-glucopyranosyl bromide was carried out in a two-phase system consisting of chloroform-1.25M aqueous potassium hydroxide solution and benzyltriethylammonium bromide as catalyst. After a simple work up, followed by column chromatography, the labdane glycosides glycosides were isolated in 30% yield.
example 3
Thiomidazolide Derivative of 3β-hydroxy-labd-14-ene-8,13-epoxy
[0055]3β-hydroxy-labd-14-ene-8,13-epoxy, was converted to its thiomidazolide (45% yield) by treatment with N,N′-thiocarbonydiimidazole (Rasmunssen, J. R. (1980) J. Org. Chem. 45, 2725-2727).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com