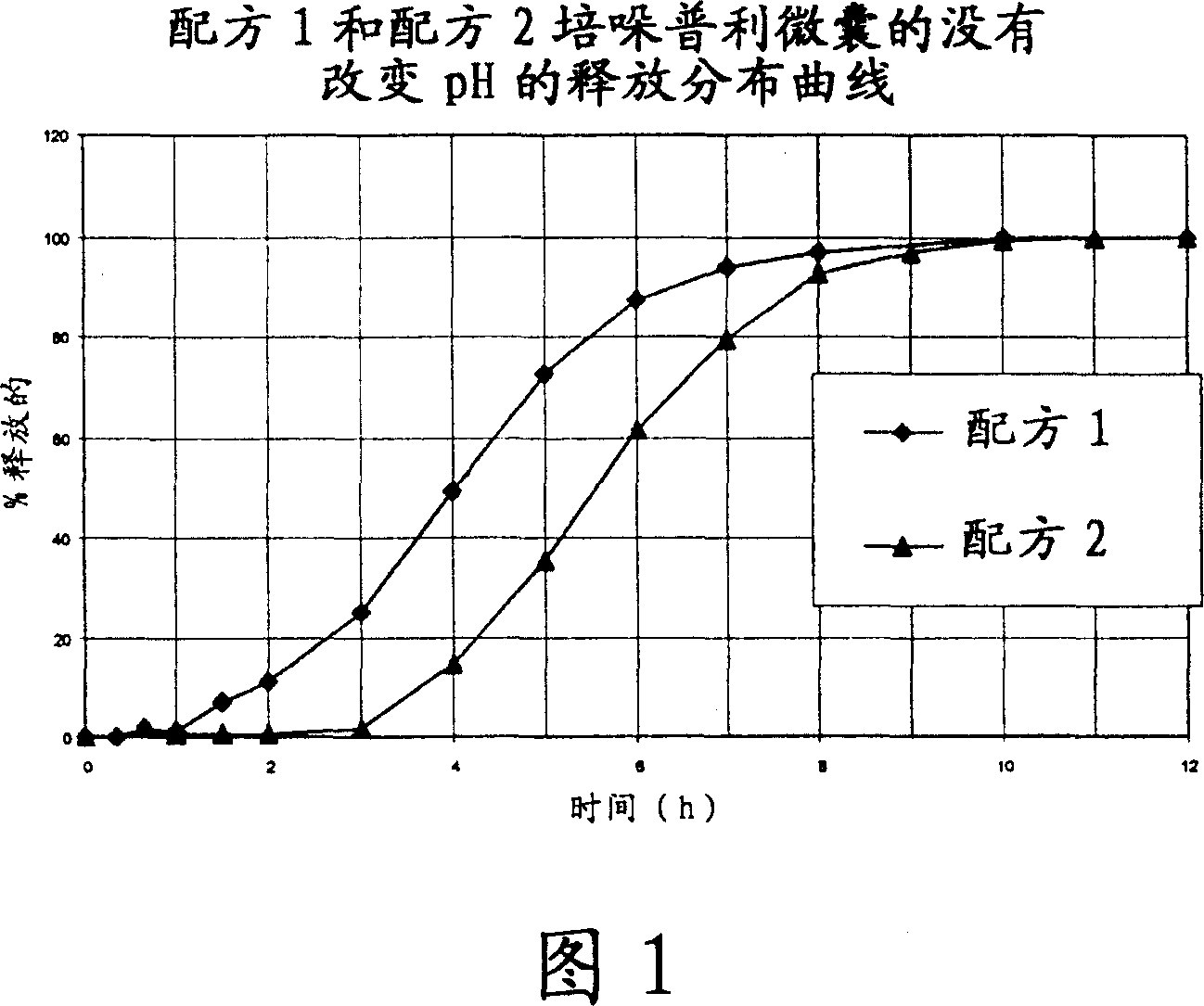
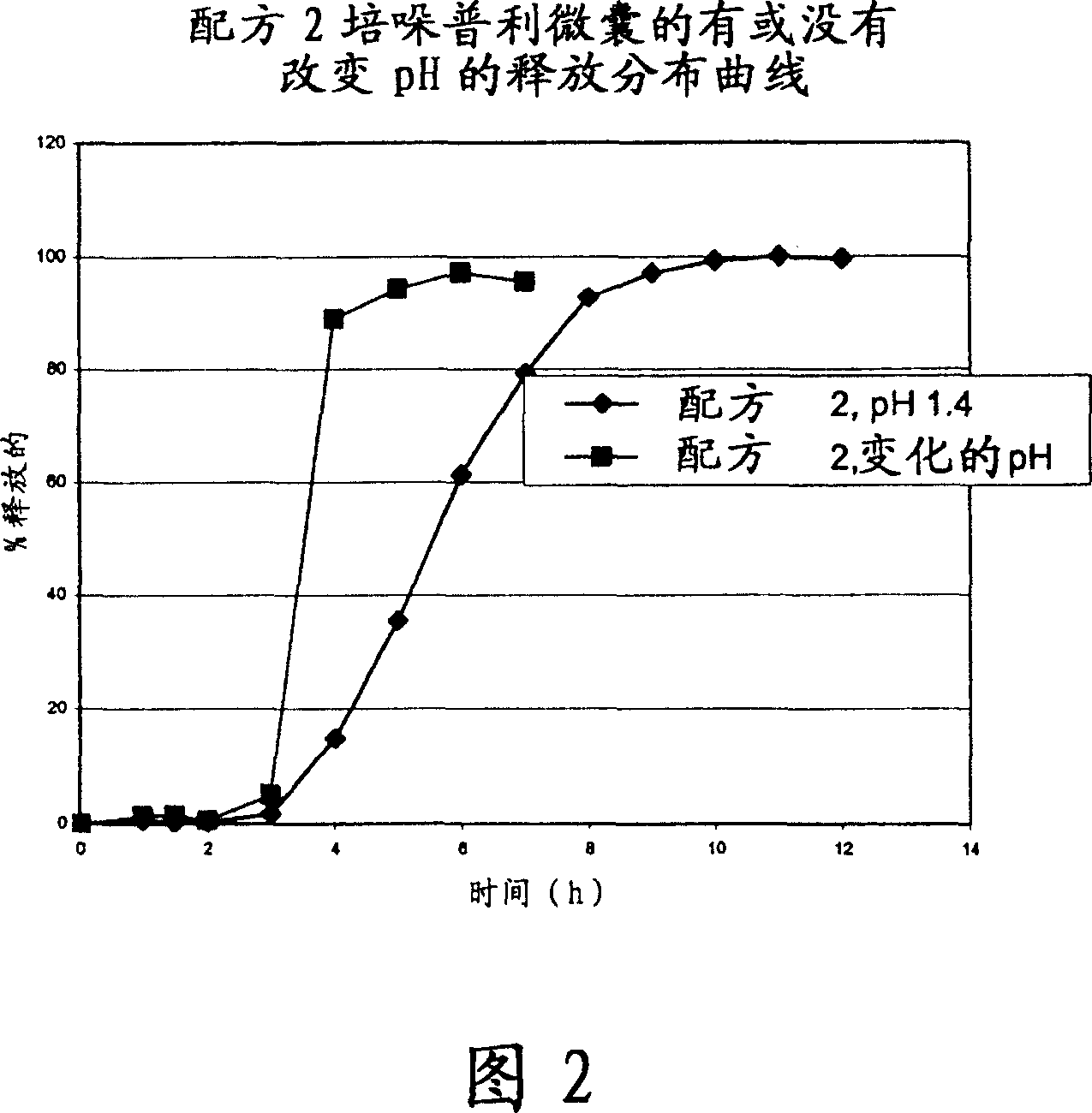
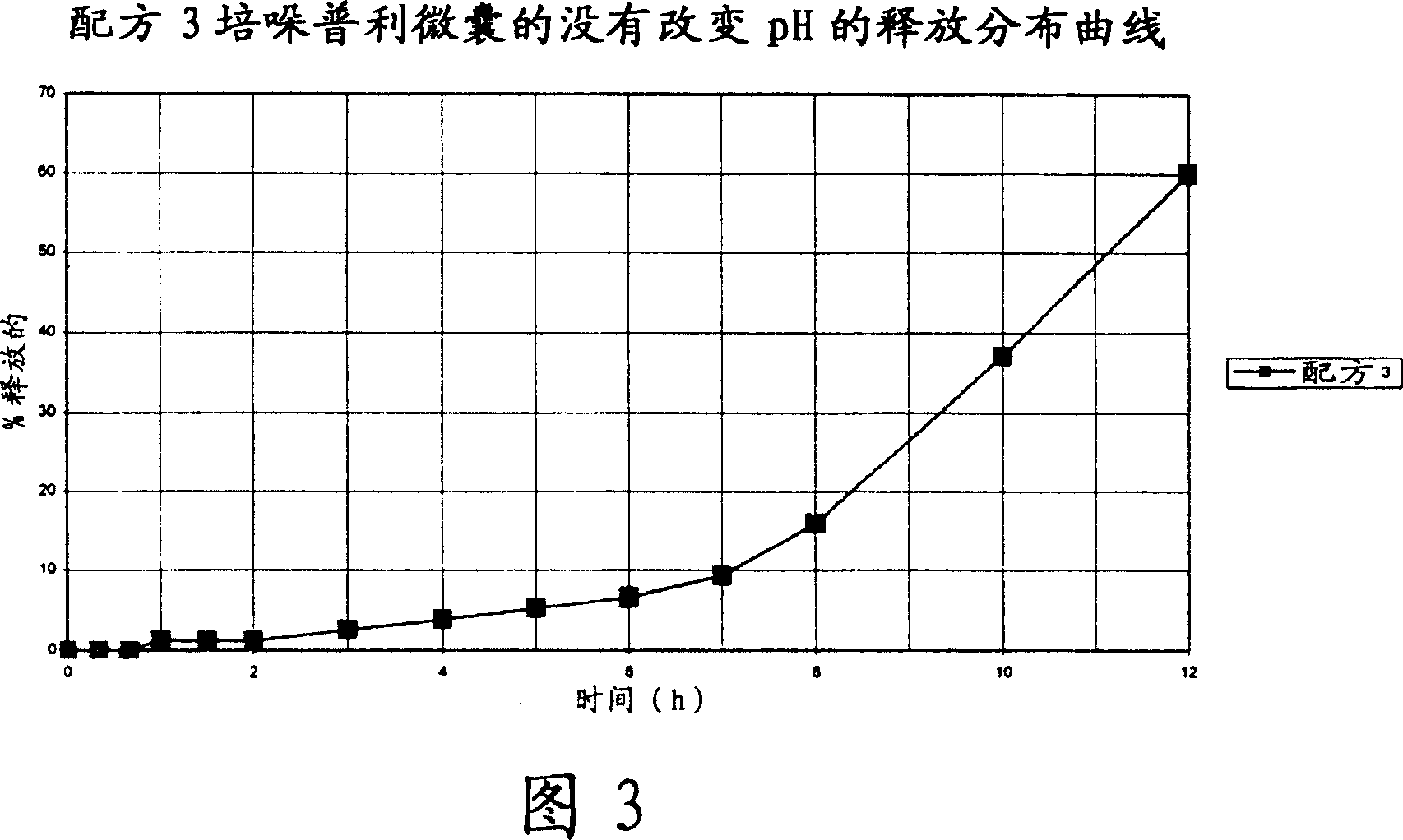
Microcapsules for the delayed, controlled release of perindopril
A technology of slow-release peridole and microparticles, which is applied in the direction of microcapsules, capsule delivery, medical preparations of non-active ingredients, etc., and can solve the problem of controlled slow-release antihypertensive dosage forms that cannot inhibit angiotensin-converting enzymes Does not include the dual mechanism of release, low AI absorption, etc., to achieve optimal compliance, treatment of arterial hypertension and heart failure, and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0113] Preparation of 1-perindopril microcapsules
[0114] Step A: Preparation of Perindopril Microparticles
[0115] 157 g of perindopril tert-butylamine salt and 17 g of hydroxypropylcellulose were dispersed or dissolved in 1300 g of acetone. The suspension was sprayed onto 1500 g of sugar microspheres with a mean diameter of 355-500 [mu]m using a Glatt GPCG3 spray coater. The film coating conditions are: product temperature: 37-39°C, spray feeding rate: 42g / min, atomization pressure: 1.8bar.
[0116] Step B: Preparation of Perindopril Microcapsules
[0117] The hydrophilic polymer A and the hydrophobic compound B were dissolved in isopropanol which had been heated to a temperature of 65-75°C. This solution was sprayed onto the perindopril microparticles prepared in step A using a Glatt GPCG3 sprayer. The film coating conditions are: product temperature: 36-41°C, spray feeding rate: 8-12g / min, atomization pressure: 1.5bar.
[0118] 2- Formulation Example
[0119] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com