Use of glucoprotein for pharmaceuticals
A fetuin and drug technology, applied in the field of new pharmaceutical uses of fetuin, can solve problems such as unclear functions and uses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0011] Experimental Example 1: Effect of Fetuin on the Infarct Size of Focal Cerebral Ischemia
[0012] 1. Animal grouping: 88 SD male rats, weighing 250g-350g, were randomly divided into 10 groups, 8 in each group. Groups 1 and 6 were model control groups, and the rest were treatment groups with different doses and administration times.
[0013] 2. Operation steps: Fetuin (fetuin) was dissolved in normal saline (1ml volume), and intravenously injected into rats at 15 minutes and 30 minutes after ischemia respectively (see Table 1).
[0014] Table 1 The effect of fetuin on the infarct size of focal cerebral ischemia
[0015]
[0016] After 24 hours of focal cerebral ischemia in rats, the animals were anesthetized and sacrificed, the brain was removed and fixed, cut into 2mm thick brain slices, immersed in 2% TTC (2,3,5-hiphenyltetrazoliam chloride) and incubated at 37°C 30 minutes. After the stained brain tissue was photographed, the cerebral infarct area was calculated ...
experiment example 2
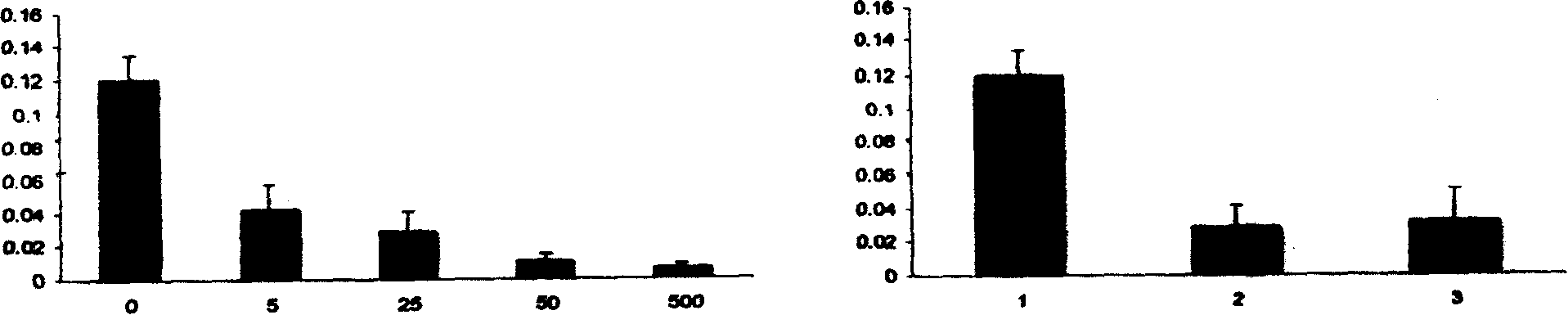
[0019] Experimental example 2: Effect of fetuin on the expression of activated microglial cells and macrophages in rats with acute ischemic brain injury
[0020] 1. Grouping of animals: 12 male SD rats, weighing 250-350 grams, were randomly divided into 2 groups: Group 1 was a fetuin treatment group, Group 1 was a model control group, 6 rats in each group.
[0021] 2. Operation steps: Fetuin (fetuin) was dissolved in normal saline (1ml volume), and intravenously injected into the treatment group rats (50mg / kg) 15 minutes after ischemia, and the model group was only injected with the same amount of normal saline. After 24 hours the animals were anesthetized and the rats (approximately 350 ml) were perfused via the left ventricle with 4% paraformaldehyde (pH 7.4). The brain was taken out, cut into 2mm thick brain slices, fixed with the same fixative solution at 4°C for 12 hours, and then put into 30% sucrose solution for 12 hours (4°C). Frozen sections (10 μm). Immunohistochem...
experiment example 3
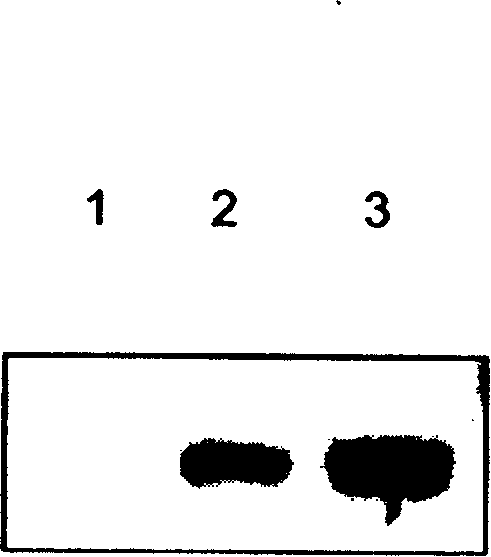
[0024] Experimental Example 3: Effect of Fetuin on the Expression of Tumor Necrosis Factor-α (TNF-α) in the Rat Ischemic Brain Injury Area
[0025] 1. Grouping of animals: the same as in Experimental Example 2;
[0026] 2. Operation steps: Fetuin (fetuin) was dissolved in normal saline (volume 1ml), and intravenously injected into the treatment group rats (50 mg / kg) within 15 minutes after ischemia, and the model group was only injected with the same amount of normal saline. Anesthetize the animal 24 hours later, perfuse the artery (about 300ml) with normal saline through the left ventricle, take out the brain, cut into 2mm brain slices, collect the ischemic injured tissue on ice, add lysis buffer to make a homogenate, and place on ice Let stand for 30 minutes and centrifuge (4°C, 2000g). The supernatant was collected, and the protein content was determined. After SDS-page electrophoresis, it was transferred to a nitrocellulose membrane, and the TNF-α content was detected by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com