Antifungal medicine composition
A technology of antifungal drugs and compositions, applied in the field of pharmacy, can solve the problems of high recurrence rate, large toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
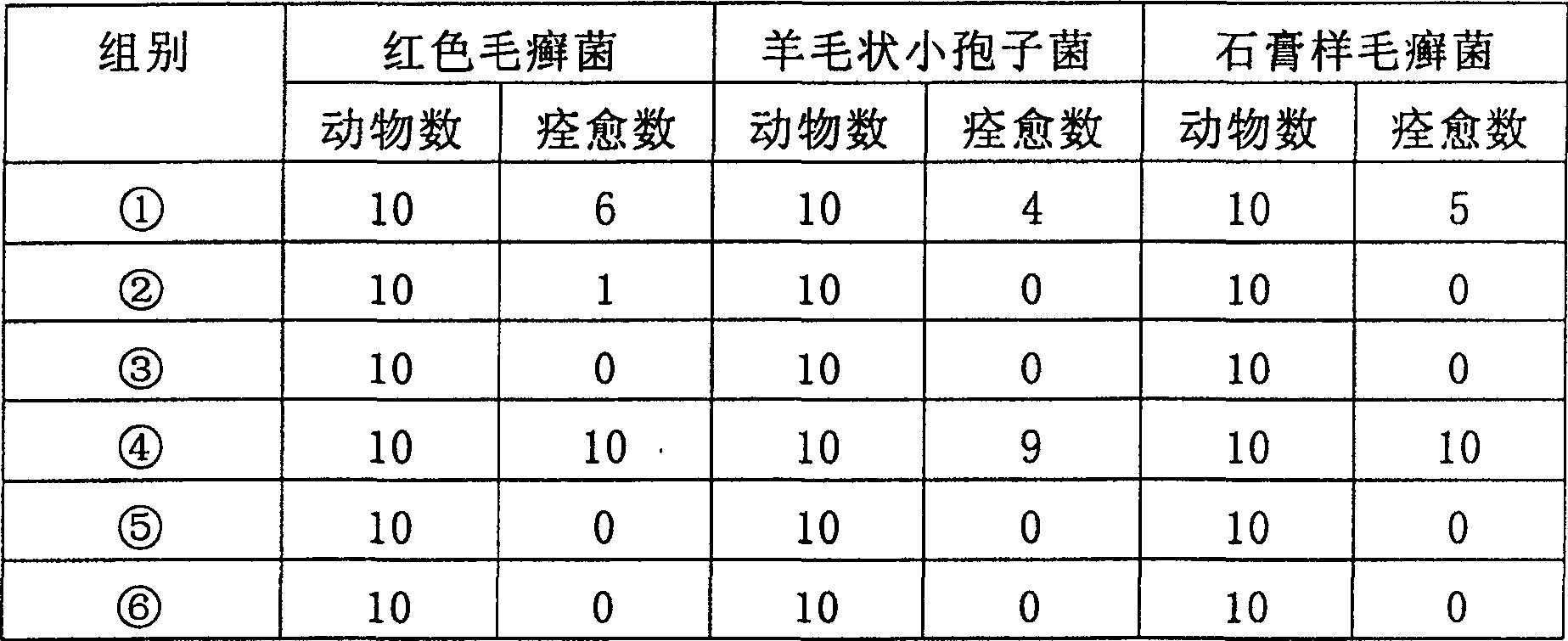
[0041] The in vitro antibacterial effect of cyclosporin A or FK-506 and fluconazole on Trichophyton rubrum, Microsporum laniformis, Trichophyton gypsum, Epidermophyton floccus, Candida albicans
[0042] The experimental method refers to the liquid-based dilution method antifungal susceptibility test protocol published by the National Committee for Clinical Laboratory Standards (NCCLS) (National Committee for Clinical Laboratory Standards. 1998.Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi.Proposedstandard M38- P. National Committee for Clinical Laboratory Standards, Wayne, Pa.)
[0043] (1) Preparation of bacterial liquid:
[0044] Add 1ml of sterilized 0.85% normal saline to the fungal slant inoculated on the slant SDA medium, gently aspirate the mixture of spores and hyphae with a sterile dropper, count on a hemocytometer, and adjust the bacterial solution with RPMI 1640 medium Concentration to 0.4-5×10 4 CFU / ml.
[0...
Embodiment 2
[0057] In vitro antibacterial effects of cyclosporin A (CsA) or FK-506 and bifonazole on Trichophyton rubrum, Microsporum laniformis, Trichophyton gypsum, Epidermophyton flocculus, Candida albicans
[0058] The same test method as in Example 1 was used to determine the antibacterial effect, except that bifonazole was used instead of fluconazole. The results are shown in Table 2.
[0059] Table 2. Antibacterial activity (MIC μg / ml) of cyclosporine A (CsA) or FK-506 combined with bifonazole (BIF)
[0060] drug Bai Nian Red hair wool plaster Flocculent CsA >64 >64 >64 >64 >64 FK-506 >64 >64 >64 >64 >64 BIF 2.5 0.125 0.125 0.5 0.25 BIF+CsA(2μg / ml) <0.125
<0.125
<0.125
0.125 <0.125
BIF+FK-506(2μg / ml) <0.125
<0.125
<0.125
0.125 <0.125
[0061] Note: Bai Nian = Candida albicans
[0062] Red hair = Trichophyton rubrum
[0063] Wool = Microsporum laniformis
[0064] Gypsum = Trichophyton gypsum
[006...
Embodiment 3
[0067] In vitro antibacterial effect of cyclosporin A (CsA) or FK-506 and terbinafine against Trichophyton rubrum, Microsporum laniformis, Trichophyton gypsum, Epidermophyton flocculus, Candida albicans
[0068] The antibacterial effect was measured by the same test method as in Example 1, except that terbinafine was used instead of fluconazole. The results are shown in Table 3.
[0069] Table 3. Antibacterial activity of cyclosporine A (CsA) or FK-506 combined with terbinafine (MIC μg / ml)
[0070] drug Bai Nian Red hair wool plaster Flocculent CsA >64 >64 >64 >64 >64 FK-506 >64 >64 >64 >64 >64 TEB 12.5 0.0025 0.01 0.005 0.005 TEB+CsA(2μg / ml) 6.25 <0.00125
0.00125 <0.00125
<0.00125
TEB+FK-506(2μg / ml) 1.5625 <0.00125
0.0025 <0.00125
<0.00125
[0071] Note: Bai Nian = Candida albicans
[0072] Red hair = Trichophyton rubrum
[0073] Wool = Microsporum laniformis
[0074] Gypsum = Trichophyton gypsu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com