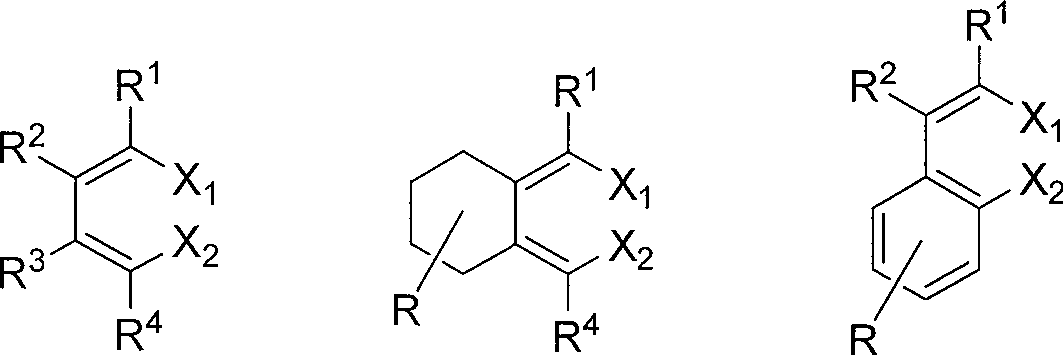
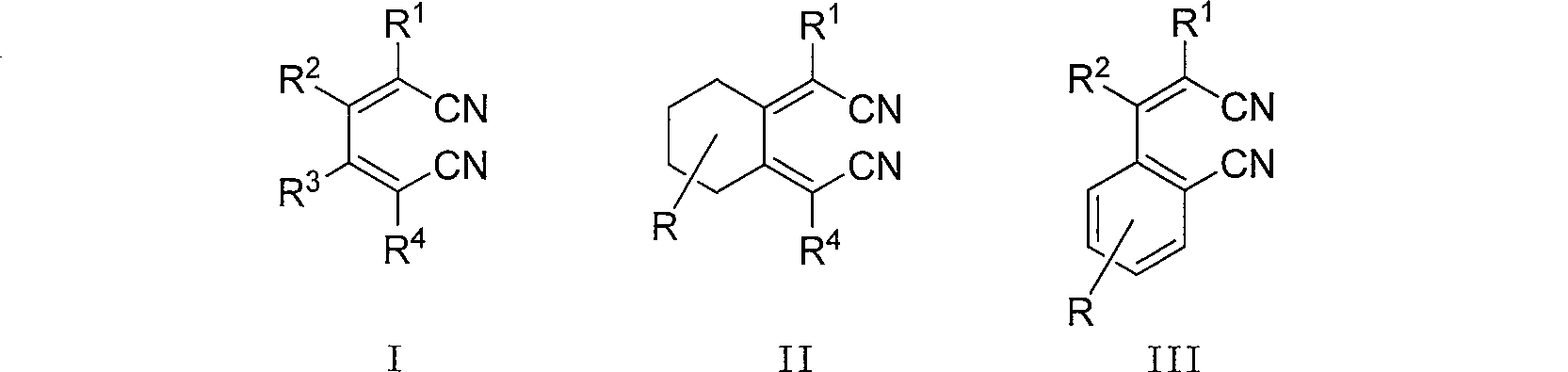
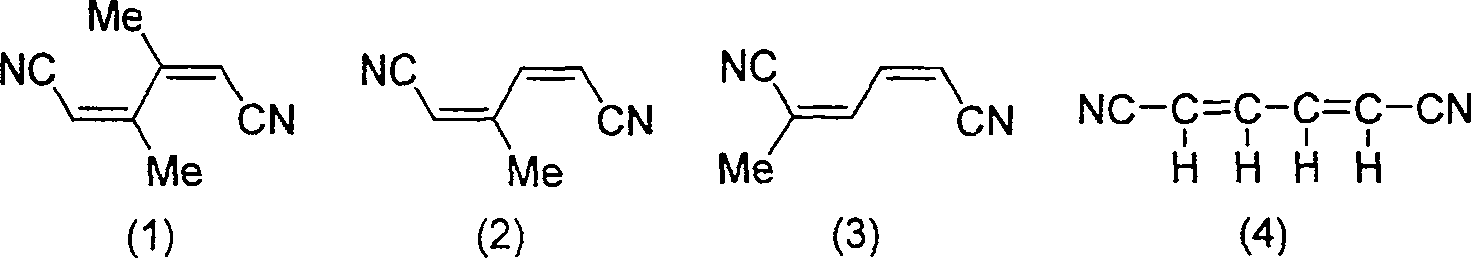
Method of synthesizing hexa-2,4-dienedinitrile from 1,4-dihalogen-1,3-butadiene and cuprous cyanide
A technology of hexadiene dinitrile and cuprous cyanide, which is applied in cyanide reaction preparation, organic chemistry and other directions, can solve the problem of low reaction selectivity, achieve high separation yield, easy availability of raw materials, experimental equipment and operation Simple and easy effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] One of the compounds of class I in the structural formula (R 1 =R 2 =R 3 =R 4 =n-Pr): Synthesis of 2,3,4,5-tetrapropyl-2,4-hexadienedionitrile:
[0026] To a 20mL round-bottomed flask, add 1mmol of 5,6-dipropyl-4,7-diiodo-4,6-decadiene and 2.5mmol of cuprous cyanide, add 5mL of DMF solvent, heat to reflux, and magnetically stir the reaction six hours. After the reaction, it was concentrated, decolorized and separated on a silica gel column, and the mixed solvent of petroleum ether:diethyl ether=10:1 was used as the eluent to obtain the pure product 2,3,4,5-tetrapropyl-2,4-hexadienedionitrile 0.248 g (purity >98%, colorless liquid), isolated yield 91%. The NMR and high-resolution mass spectrometry data of this compound are as follows: 1 H NMR (300MHz, CDCl 3 ): δ 0.96-1.03(m, 12H), 1.42-1.49(m, 4H), 1.61-1.73(m, 4H), 2.28-2.33(m, 8H). 13 C NMR (75MHz, CDCl 3 ): delta 13.02, 13.85, 20.59, 20.9231.47, 32.51, 114.27, 118.09, 155.70. HRMS: calcd for C 18 H 28 N 2...
Embodiment 2
[0028] The second compound of class I in the structural formula (R 1 =R 2 =n-Et, R 3 =R 4 =Ph): Synthesis of 2,3-diethyl-4,5-diphenyl-2,4-hexadienedionitrile
[0029] The synthetic route is basically the same as above. The starting material dihalogen compound of this synthesis is 3-ethyl-1,2-diphenyl-1,4-diiodo-1,3-hexadiene. 0.300 g of pure product (purity >98%, colorless solid, melting point: 105-106° C.) was obtained in 96% isolated yield. The NMR, high-resolution mass spectrometry and elemental analysis data of this compound are as follows: 1 H NMR (300MHz, CDCl 3 ): δ 1.09(t, J=7.5Hz, 3H), 1.34(t, J=7.5Hz, 3H), 2.26(br, 2H), 2.50(q, J=7.5Hz, 2H), 7.06-7.31( m, 10H). 13 C NMR (75MHz, CDCl 3 ): delta 12.06, 12.52, 23.65, 24.28, 115.88, 117.28, 118.38, 118.53, 128.64, 128.78, 129.03, 129.38, 129.65, 129.80, 133.15, 134.42, 154.83, 156.72 22 H 20 N 2 312.1627, found 312.1617.Anal.Calcd for C 22 H 20 N 2 : C, 84.64; H, 6.54, N, 8.97. Found: C, 84.58; H, 6.45, N...
Embodiment 3
[0031] The third compound of class I in the structural formula (R 1 =R 4 =n-Bu,R 2 =R 3 =H): Synthesis of 2,5-dibutyl-2,4-hexadienedionitrile
[0032] The synthetic route is basically the same as above. The starting material dihalogen compound of this synthesis is 5,8-dibromo-5,7-dodecadiene. The pure product 0.182 g (purity >98%, colorless liquid) was obtained in 84% isolated yield. The NMR and high-resolution mass spectrometry data of this compound are as follows: 1 HNMR (300MHz, CDCl 3 ): δ 0.95(t, J=7.5Hz, 6H), 1.31-1.44(m, 4H), 1.54-1.64(m, 4H), 2.36(t, J=7.5Hz, 4H), 6.97(s, 2H) ). 13 C NMR (75MHz, CDCl 3 ): δ 13.68, 21.92, 30.06, 34.37, 116.74, 120.60, 138.42. HRMS: calcd for C 14 H 20 N 2 216.1627, found 216.1625.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com