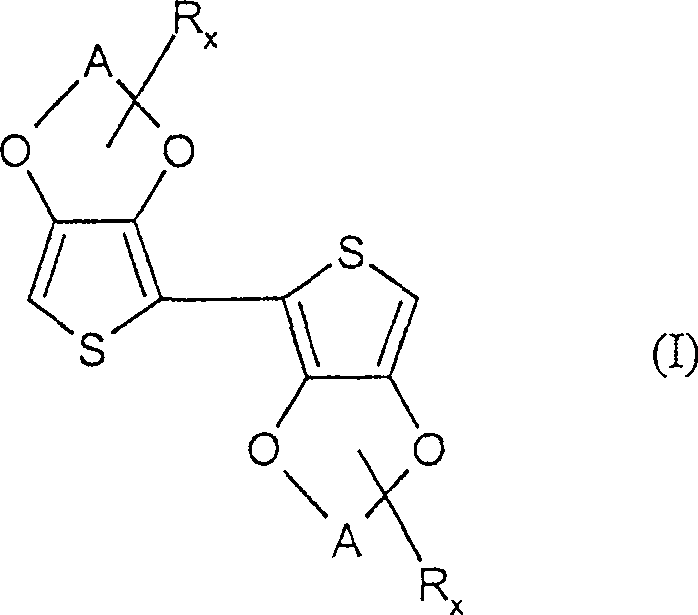
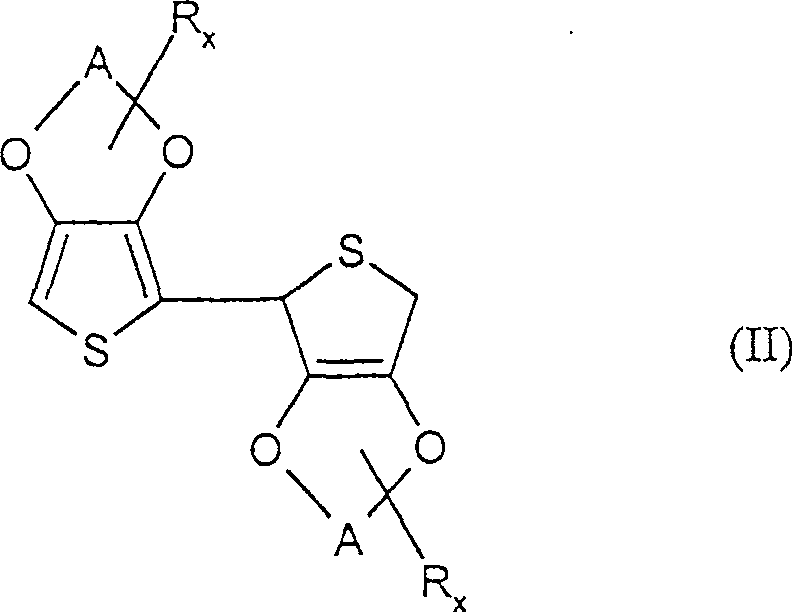
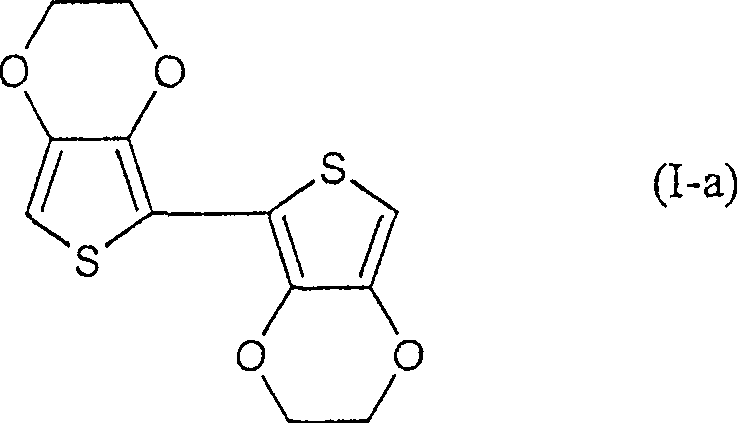
Preparation of 2,2'-di(3,4-ethylenedioxy-thiophene)
A C6-C14, alkyl technology, applied in the field of preparation 2, can solve the problems of increased reaction time, air and moisture sensitivity, no mention of having alkylene bridges, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Embodiment 1: prepare 2,2'-two (3,4-ethylenedioxythiophene) (I-a) (the present invention)
[0106]
[0107] 28.64 g (0.1 mol) of EDT dimer (II-a) were dissolved in 500 ml of xylene. To this solution was added 24.59 g (0.1 mol) of chloranil. The mixture was heated to reflux at 140°C for 10 hours. After cooling, the precipitated crystals were filtered off with suction, and the crystals and the mother liquor were worked up further.
[0108] The mother liquor was washed 4 times with 50 ml of 4% potassium hydroxide solution each time, and finally washed with water until neutral. The solution was concentrated by evaporation and dried under high vacuum, yielding 15.8 g of crude product as green crystals (fraction A).
[0109] The suction-filtered crystals were slurried with 150 ml of xylene and 100 ml of 4% strength potassium hydroxide solution, and the insoluble fraction (5.5 g) was removed by filtration. The mother liquor was worked up in the same manner as above, a...
Embodiment 2
[0114] Embodiment 2: prepare 2,2'-bis(3,4-ethylenedioxy with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone base thiophene) (I-a)
[0115] 8.53 g (30 mmol) of EDT dimer (II-a) were dissolved in 150 ml of xylene. To this solution was added 7.15 g (31.5 mmol) of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. The mixture was heated to reflux at 140°C for 24 hours. After cooling, the mixture was extracted with 4% potassium hydroxide solution until the potassium hydroxide solution remained colorless. Finally the mixture was washed with water until neutral. The solution was concentrated by evaporation and dried under high vacuum, yielding 3.68 g (43.5% of theory) of crude product as a beige residue. The crude product was purified by column chromatography on silica gel eluting with dichloromethane.
[0116] 1.32 g (15.6% of theory) of pure 2,2'-bis(3,4-ethylenedioxythiophene) (I-a) were isolated. Melting point: 207-209°C.
[0117] 1 H NMR spectrum (400MHz, CDCl 3 ) is the same as ...
Embodiment 3
[0118] Example 3: Preparation of dimethyl-2,2-3,3'-tetrahydro-5,5'-dithieno[3,4-b]1,4-dioxin (II-b)
[0119]
[0120] 0.85 g (2.7 mmol) of methyl-EDT dimer (II-b) was dissolved in 50 ml of xylene. To this solution was added 0.668 g (2.7 mmol) of chloranil. The mixture was heated to reflux at 140°C for 14 hours. After cooling, the mixture was diluted with dichloromethane and extracted with 4% potassium hydroxide solution until the potassium hydroxide solution remained colorless. Finally, the mixture is washed with water until neutral. The solution was concentrated by evaporation and dried under high vacuum, yielding 0.48 g (56.7% of theory) of crude product (I-b) as residue. The product was purified by column chromatography on silica gel eluting with dichloromethane. Pure yield 0.38 g (44.8% of theory).
[0121] 1 H NMR spectrum (400MHz, CDCl 3 , ppm): 6.24, 6.235, 6.23 (3s, 2H), 4.39 (with more split q, 3 J=6.56Hz) and 4.32 (q with more splits, 3 J=6.56Hz, all ar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com