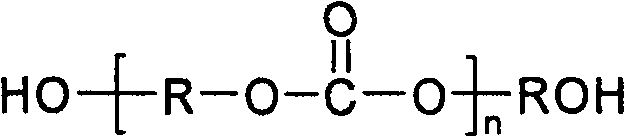Method of preparing polycarbonate dihydric alcohol
A technology of polycarbonate di- and diethyl carbonate is applied in the field of preparation of polycarbonate diol, and can solve the problems of difficulty in removal and difficult control of technological process, so as to achieve uninhibited reactivity, stable reactivity, and high technological progress. The effect of easy control of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A) In a 1L glass flask, add 427g of diethyl carbonate, 625g of 1,6-hexanediol and 0.084g of tetraisopropyl phthalate, stir and react at 200°C for 3 hours, then stir at 100mmHg Under the pressure, after continuing to react for 5.5 hours, obtain polycarbonate diol 537g containing transesterification catalyst.
[0021] B) Take 100g of the above product in a 500mL reaction flask, add 3g of water at 120°C, stir for 1 hour to deactivate the catalyst, remove excess water under reduced pressure, and obtain polycarbonate without transesterification catalyst Ester diol.
[0022] Polyurethane reaction:
[0023] Add 100 g of the above-mentioned polycarbonate diol that has undergone deactivation treatment into a 500 mL reaction flask, heat it to 70 ° C, then add MDI, take a sample after reacting for 1 hour, and measure the conversion rate of MDI in the system. The results are shown in Table 1 .
Embodiment 2
[0025] 100 g of polycarbonate diol containing transesterification catalyst synthesized by the method in Example 1, added 8.78 mg of tributyl phosphite, heated to 120° C. under continuous stirring, heated and stirred at this temperature for 3 hours, cooled and discharged . Carry out polyurethane reaction with the method of embodiment 1, take a sample after 1 hour, measure the conversion rate of MDI in the system, the results are shown in Table 1.
Embodiment 3
[0027] A) In a 1L glass flask, add 490 g of 1,5-pentanediol, 410 g of diethyl carbonate, 0.08 g of tetraisopropyl phthalate as a transesterification catalyst, stir and react for 4 hours at 150° C., and then Under the pressure of 100 mmHg, after continuing to react for 5.5 hours, the polycarbonate diol containing the transesterification reaction catalyst was obtained.
[0028] B) Take 100g of the above product in a 500mL reaction flask, add 3g of water at 120°C, stir for 1 hour to deactivate the catalyst, remove excess water under reduced pressure, and obtain polycarbonate without transesterification catalyst Ester diol.
[0029] Polyurethane reaction:
[0030] Add 100 g of the above-mentioned polycarbonate diol that has undergone deactivation treatment into a 500 mL reaction flask, heat it to 70 ° C, then add MDI, take a sample after reacting for 1 hour, and measure the conversion rate of MDI in the system. The results are shown in Table 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com