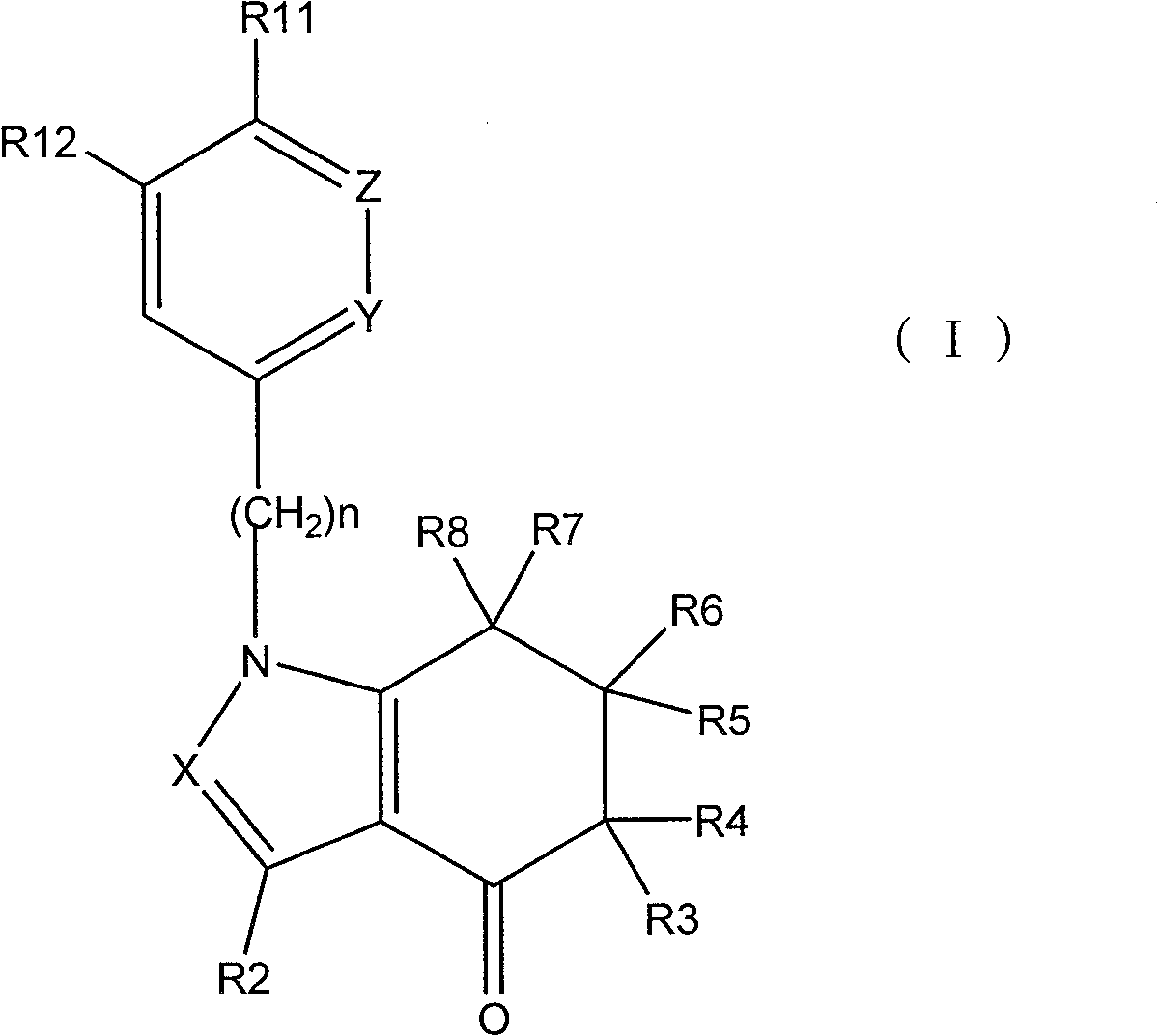
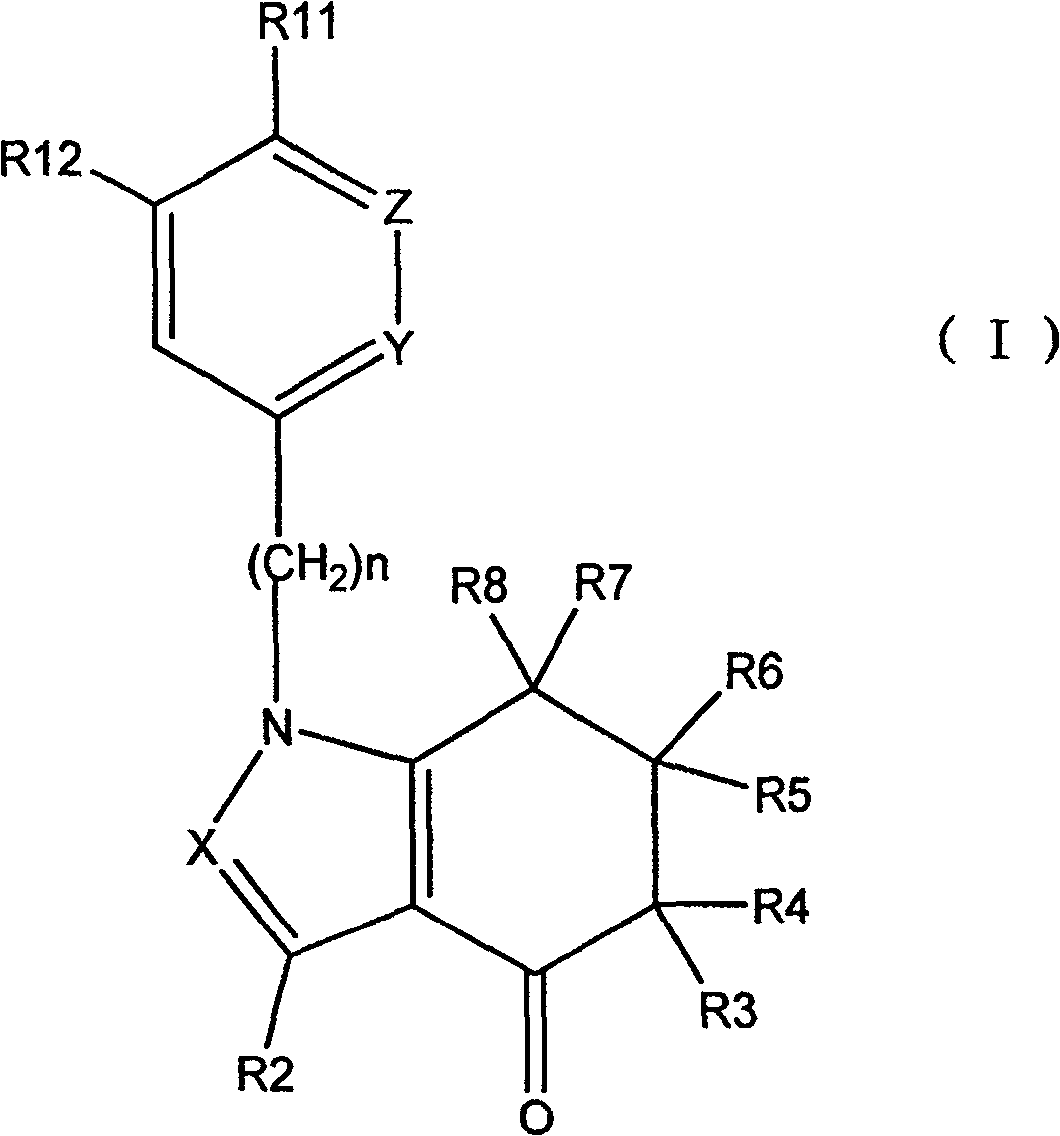
Tetrahydro-indolone derivative and tetrahydro-indazolone derivatives and their use
A compound, alkyl technology, applied in the field of derivatives of tetrahydroindolinone, can solve problems such as not mentioned
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
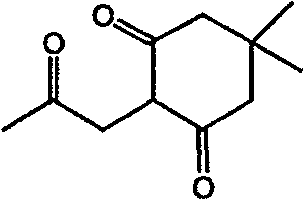
[0054] 5,5-Dimethyl-2-(2-oxo-propyl)-1,3-cyclohexanedione (5,5-dimethyl-2-(2-oxo-propyl)-cyclohexane-1,3- dione) synthesis. (Intermediate I)
[0055]
[0056] In a reaction flask, add 5,5-dimethylcyclohexane-1,3-dione (3.5 g, 25 mmol) dissolved in THF (250 mL) and sodium hydride (1g, 40mmol), under ice-bath cooling, chloroethylketone (2.3g, 25mmol) was added dropwise in the reaction flask, after stirring at room temperature for 2h, water (400mL) was added, and the reaction product was extracted with dichloromethane, and after washing with water, no dried over magnesium sulfate. After removing the desiccant, the solvent was evaporated under reduced pressure to obtain a colorless liquid, 2.8 g, with a yield of 57%. This compound was used in the following reaction without purification.
example 2
[0058] 5,5-Dimethyl-2-(2-propionyl)-1,3-cyclohexanedione (5,5-dimethyl-2-(1-methyl-2-oxo-ethyl)-cyclohexane-1 , 3-dione) synthesis. (Intermediate II)
[0059]
[0060] Add 5,5-dimethylcyclohexane-1,3-dione (5,5-dimethylcyclohexane-1,3-dione) (3.5 g, 25 mmol) dissolved in THF (200 mL) and 2N NaOH ( 15mL, 30mmol), under ice bath cooling, 2-chloropropionaldehyde dimethyl acetal (2-chloro-1, 1-dimethoxypropane) (3.5g, 25mmol) THF (50mL) was added dropwise in the reaction flask, at room temperature After stirring for 2 hours, 1N HCl (200 mL) was added and stirred for 1 hour. The reaction product was extracted with ethyl acetate, washed with saturated sodium chloride water and dried over anhydrous sodium sulfate. After removing the desiccant, the solvent was evaporated under reduced pressure to obtain 1.9 g of a colorless liquid with a yield of 39%. This compound was used in the following reaction without purification.
example 3
[0062] Synthesis of 2-acetyl-5,5-dimethyl-1,3-cyclohexanedione (2-acetyl-5,5-dimethyl-cyclohexane-1,3-dione). (Intermediate III)
[0063]
[0064] Acetyl chloride (8.8 mL) was added dropwise to a solution of 5,5-dimethyl-1,3-cyclohexanedione (16.8 g, 120 mmol) and pyridine (9.2 mL) in chloroform (400 mL). After the addition was complete, the reaction was stirred at room temperature for an additional 1.5 hours. The reaction solution was extracted with chloroform, washed with 0.1N hydrochloric acid, water, saturated aqueous sodium bicarbonate and saturated aqueous sodium chloride, and dried overnight over sodium sulfate.
[0065] The crude product obtained as a colorless liquid after filtration and solvent removal was added to a solution of anhydrous aluminum chloride (32 g, 240 mmol) in chloroform (400 mL) at 0 °C. After the addition was complete, the reaction was stirred at 0°C for 10 minutes, then at room temperature for 2 hours. The reaction solution was poured into a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com