Application of glycosamine for preparing medicine to relieve chronic pain
A technology for glucosamine and chronic pain, which is applied in the application field of glucosamine in the preparation of drugs for relieving chronic pain, and can solve the problem that non-steroidal anti-inflammatory drugs destroy internal environment stability, and cause drug tolerance, dependence and addiction. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
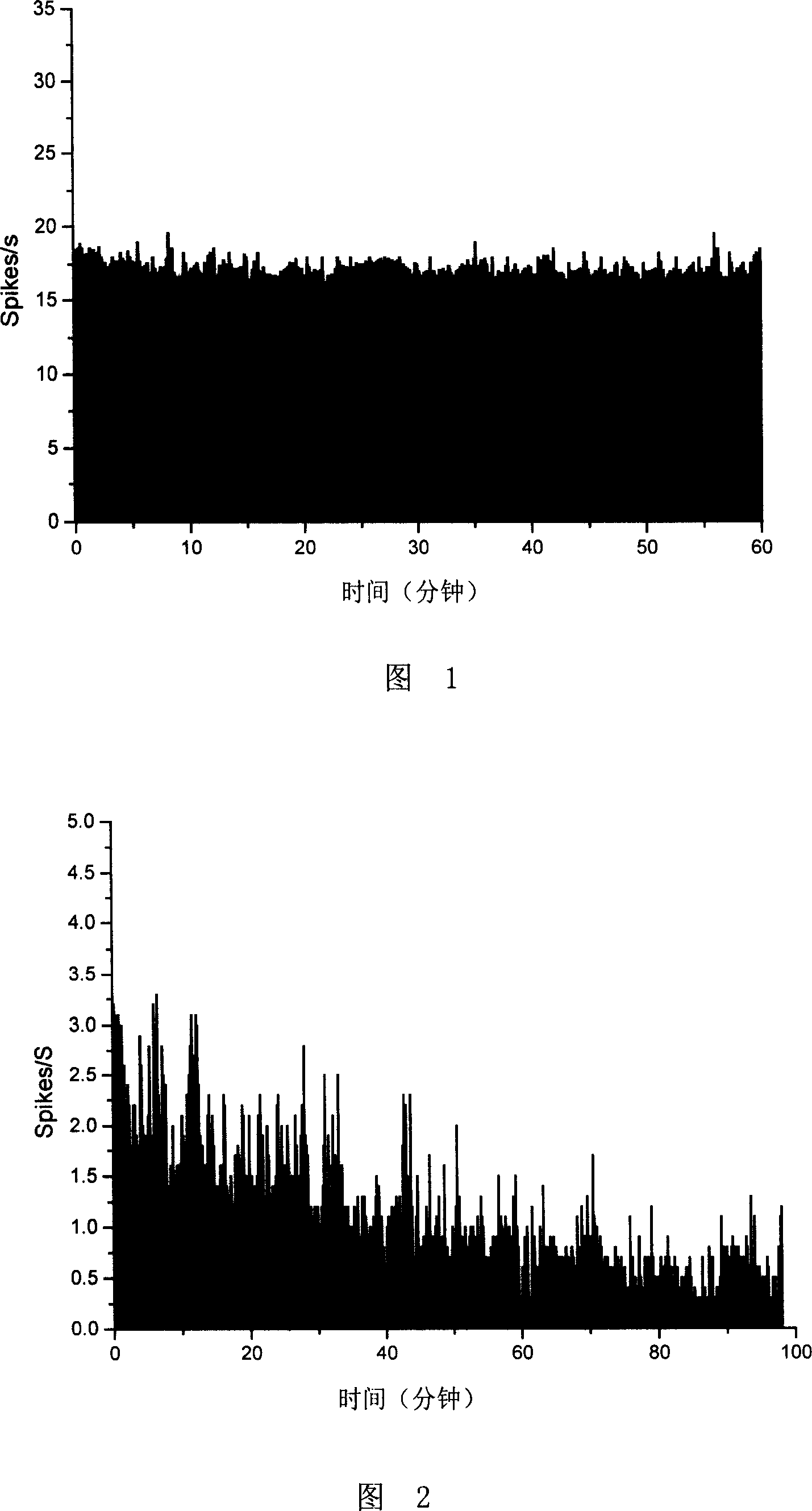
[0018] Example 1. Detection of the influence of glucosamine on the ectopic spontaneous discharge of injured nerves in the CCI rat model
[0019] Injured nerves in animal models of chronic constriction injury (CCI) can produce ectopic spontaneous discharges, and the frequency of discharges represents the degree of pain in animals. In this embodiment, the following methods are used to detect the influence of glucosamine on the ectopic spontaneous discharge produced by the injured nerve in the CCI rat model:
[0020] 1. Construction of CCI rat model
[0021] Sprague-Dawley (SD) adult rats (about 200 g in body weight, purchased from Beijing Vidolihua Experimental Animal Company) were injected intraperitoneally with 1% sodium pentobarbital at a dose of 40 mg / kg to make them anesthetized. Expose the sciatic nerve for about 1 cm, and then use No. 0 chrome catgut (No. 0-4, purchased from Davis&Geck Co.) to lightly ligate every 1 mm above the trifurcation. A total of 4 ligation points...
Embodiment 2
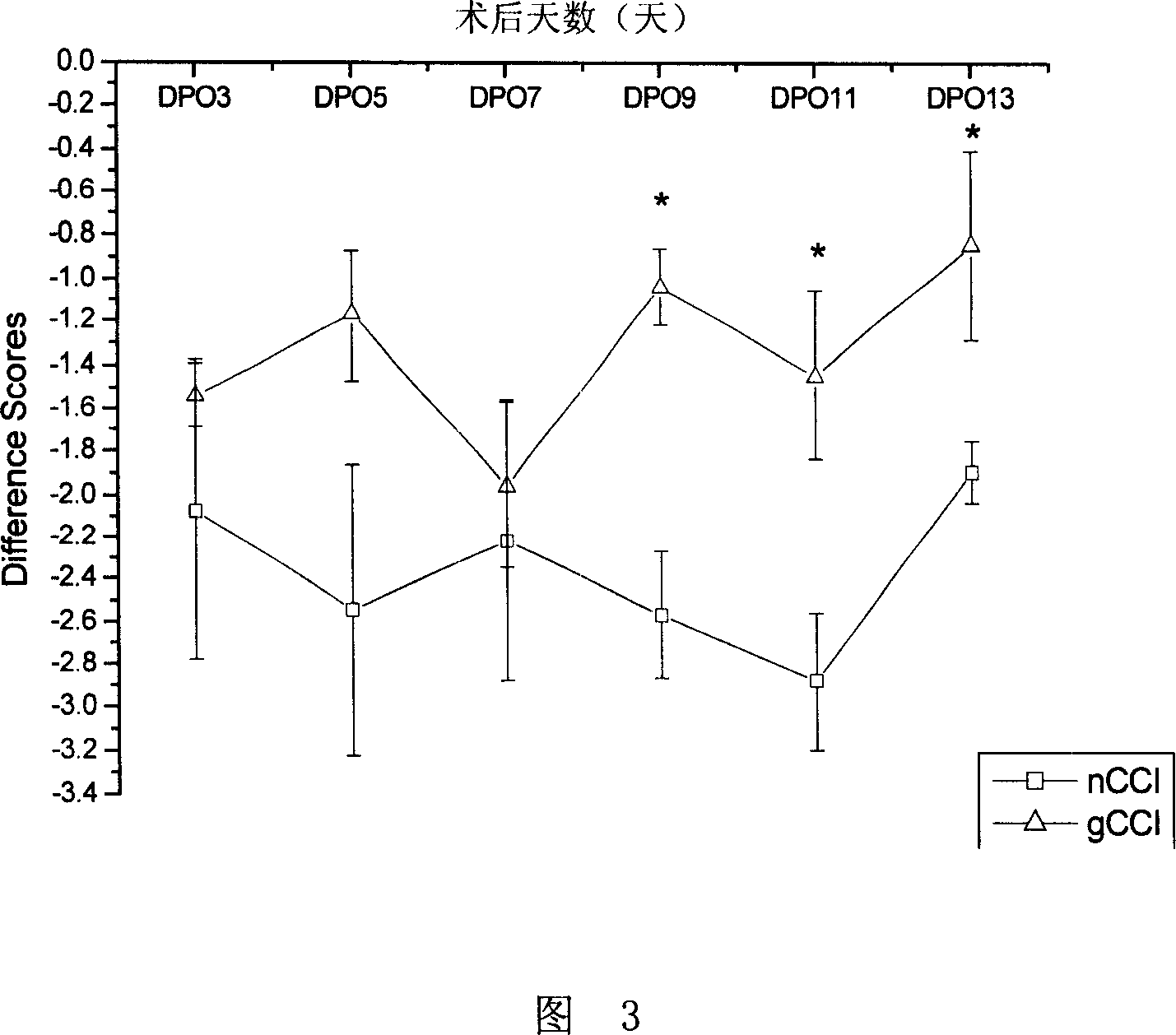
[0025] Example 2. Detection of the influence of glucosamine on the sensitivity to heat and pain stimulation of the damaged lateral foot of the CCI model
[0026] Detect the impact of glucosamine on the thermal pain stimulation sensitivity of the injured side foot of the CCI model with the following method: first, construct the CCI rat model with the same method as in Example 1; then divide the CCI rats into an experimental group and a control group, There were 10 rats in each group. The control group: fed 1 mL of pure water by intragastric administration every day after the operation; the experimental group: fed 1 mL of glucosamine with a concentration of 7.5 mg / mL (purchased from Beijing Chemical Reagent Company, Analytical Pure), the two groups of animals were tested for the sensitivity to heat and pain stimulation of the injured lateral foot on the 3rd, 5th, 7th, 9th, 11th, and 13th days after the operation, and the physiological indicators of the experimental rats were test...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com