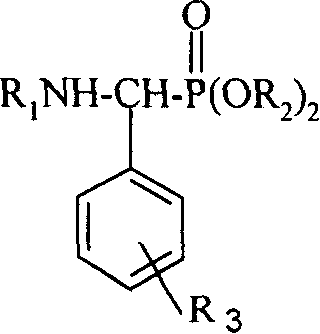Rosinyl diterpene modified alpha - phosphoramidate, preparation method, and application for anti tumors
A technology of rosin-based diterpene amine and rosin-based tricyclic diterpene, applied in the field of derivatives of rosin resin acid compounds, to achieve the effects of improving biological activity, increasing added value, and improving fat solubility
Inactive Publication Date: 2010-09-08
JIANGSU QIANGLIN BIO ENERGY
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
(Document 1: Feio S.S.; Giganteb, C.R.; Marcelo-Curto M.J.Method on multiwell plates for the evaluation of the antimicrobial activity of resin acid derivatives[J], J.ofMicrobio.Methods.1997, 28, 201-206; Document 2: Hu Deyu, Song Baoan , Zhang Guoping, et al., Synthesis and crystal structure of O, O′-di-n-butyl-α-(4-trifluoromethylanilino)-2-fluorophenylphosphonate under ultrasonic irradiation[J], Organic Chemistry , 2005, 25(7), 854-858.) People try to use various synthetic methods to prepare N-terminal (nitrogen terminal), C-terminal (carbon terminal) and P-terminal (phosphorus terminal) with different substituents New derivatives of amino phosphonates, in order to find compounds with high biological activity, the N-terminal introduction of tricyclic diterpenes and groups with multiple chiral centers has not been reported in the literature
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Login to View More
Abstract
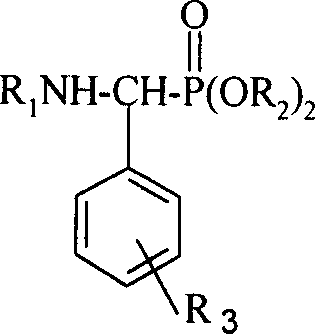
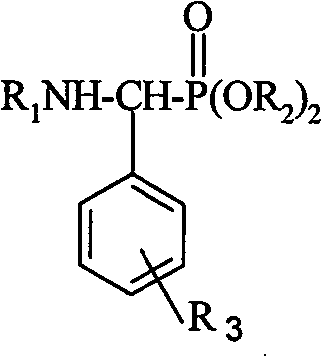
This invention relates to a method for preparing roinyl diterpene modified alpha-aminophosphate, and its anti-tumor application. The method comprises: reacting roinyl amine diterpenoid with substituted benzaldehyde and phosphite at a mol ratio of 1 :( 1-1.05) :( 1-1.1) by solvent synthesis, one-pot synthesis or solvent-free synthesis. The inroduction of roinyl diterpene into aminophosphate can largely improve the liposolubility and bioactivity of the compound. R1 structure comes from natural product rosin, thus has low toxicity.
Description
technical field The invention relates to a derivative of rosin resin acid compound, a preparation method and its application as a medicine, in particular to a rosin-based diterpene modified α-phosphoramidate, a preparation method and an antitumor application. Background technique Amino phosphonates, as phosphorous analogs of amino acid esters, have a wide range of herbicidal, bactericidal and plant growth regulating activities. Certain amino phosphonates also have biological activities such as anticancer and antitumor and are used as starting materials to synthesize a series of phosphorus peptide. Therefore, research on the synthesis and biological activity of aminophosphonate derivatives has aroused widespread interest. (Document 1: Feio S.S.; Giganteb, C.R.; Marcelo-Curto M.J.Method on multiwell plates for the evaluation of the antimicrobial activity of resin acid derivatives[J], J.ofMicrobio.Methods.1997, 28, 201-206; Document 2: Hu Deyu, Song Baoan , Zhang Guoping, e...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): A61P35/00A61K31/662C07F9/40
Inventor 宋湛谦饶小平商士斌高宏姚绪杰
Owner JIANGSU QIANGLIN BIO ENERGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com