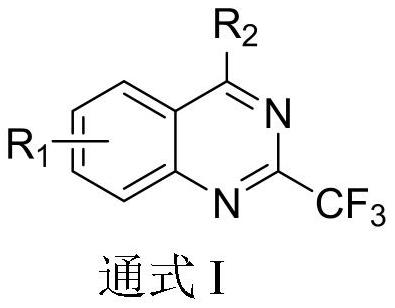
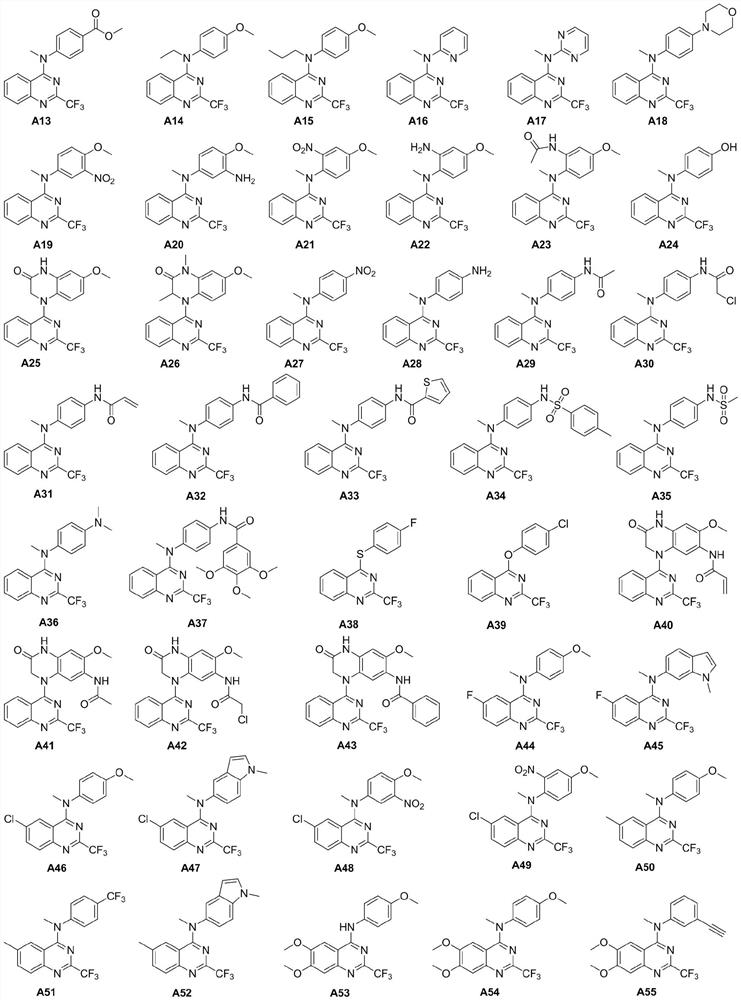
2-trifluoromethyl-4-aminoquinazoline compound and application thereof
A technology of aminoquinazoline and trifluoromethyl, which is applied in the field of quinazoline compounds to achieve the effects of improving druggability, increasing biological activity, and increasing the probability of interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the synthesis of intermediate M3:
[0031]
[0032] Take 12g (14.7mmol) of anthranilamide S (14.7mmol) and 2.44g (17.6mmol) of potassium carbonate in a reaction flask, add 15mL of anhydrous dichloromethane, and slowly drop in 2.17mL (15.4mmol) of trifluoroacetic anhydride under stirring in an ice-water bath , continue to stir until the reaction is complete, add 15 mL each of dichloromethane and water, extract, dry the organic phase with anhydrous magnesium sulfate, and evaporate to dryness under reduced pressure. The resulting crude product is purified by silica gel column chromatography to obtain 2.98 g of white solid M1, and the yield is 87.4%. 1 H NMR (600MHz, DMSO-d 6)δ13.70(s,1H),8.55(s,1H),8.40(dd,J=8.3,0.7Hz,1H),8.03(s,1H),7.97(dd,J=7.9,1.3Hz,1H ), 7.63 (t, J=7.8Hz, 1H), 7.32 (t, J=7.6Hz, 1H). Take 12g (8.60mmol) of intermediate M and 483mg (8.60mmol) of potassium hydroxide in a reaction flask, add 20mL of acetonitrile, stir at 80°C for 4h unt...
Embodiment 2
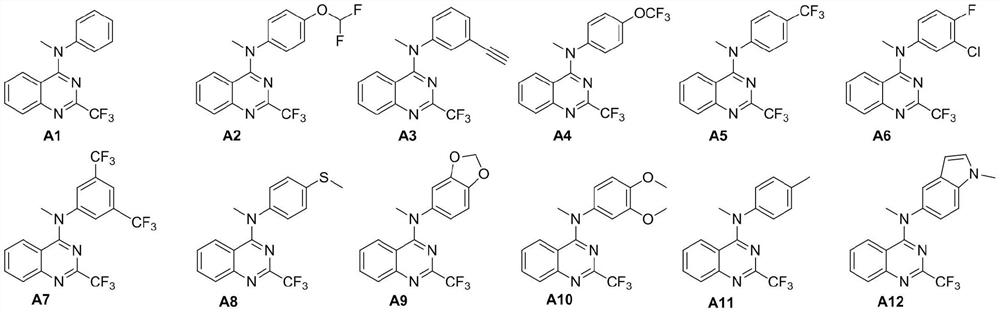
[0033] Embodiment 2: the synthesis of compound A1:
[0034]
[0035] Take 120mg (1.29mmol) of aniline and 330mg (1.42mmol) of intermediate M3 in a reaction flask, add 4mL of isopropanol and 218μL (2.58mmol) of concentrated hydrochloric acid, stir at 80°C for 6h until the reaction is complete, and solids precipitate out after the reaction solution is cooled , suction filtration, the filter cake was washed with petroleum ether, and dried to obtain 351 mg of white solid M4, with a yield of 94.1%. 1 H NMR (600MHz, DMSO-d 6 )δ10.41(s,1H),8.80(d,J=8.2Hz,1H),7.98(t,J=7.5Hz,1H),7.95–7.89(m,3H),7.79(t,J=7.5 Hz, 1H), 7.43 (t, J = 7.9Hz, 2H), 7.19 (t, J = 7.4Hz, 1H). Take 180 mg (622 μmol) of intermediate M4 in a reaction flask, add 5 mL of anhydrous DMF, add 30 mg (1.24 mmol) of sodium hydrogen in an ice-water bath with stirring, stir until no bubbles are generated, then inject 77 μL (1.24 mmol) of methyl iodide, and slowly rise to Stir at room temperature overnight until the reac...
Embodiment 3
[0036] Example 3: Synthesis of Compound A2: Referring to the preparation of A1 in Example 2, 4-difluoromethoxyaniline was used instead of aniline to obtain a white solid with a yield of 85.3%. 1 H NMR (600MHz, DMSO-d 6 )δ7.91(d,J=8.3Hz,1H),7.78(ddd,J=8.3,7.0,1.2Hz,1H),7.50–7.44(m,2H),7.44–7.17(m,4H),6.97 (d,J=8.0Hz,1H),3.60(s,3H). 13 C NMR (151MHz, DMSO-d 6 )δ162.14,151.19(q,J=34.7Hz),150.93,150.11(t,J=3.3Hz),144.18,133.85,129.23,128.38,127.59,126.34,120.81,120.42(q,J=275.6Hz),118.40 ,116.69,116.20,114.98. 19 F NMR (565MHz, DMSO-d 6 )δ-69.40,-82.46.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com