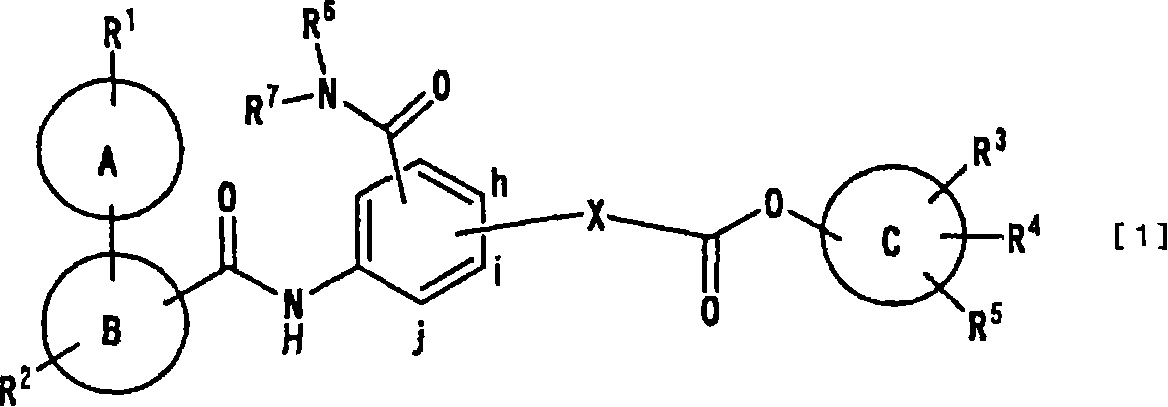
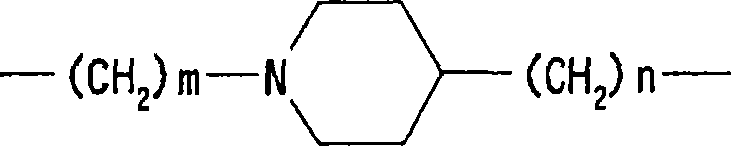
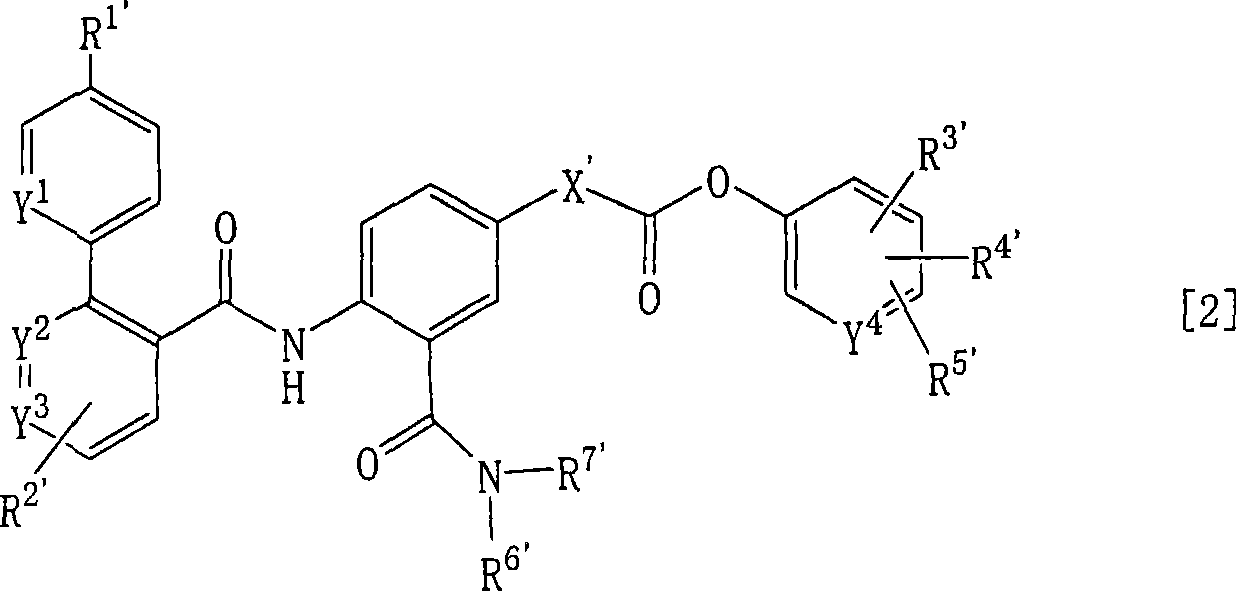
Ester derivatives and medicinal use thereof
An ester compound, C1-C6 technology, applied in pharmaceutical formulations, medical preparations containing active ingredients, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0700] The present invention is explained in detail by the following examples, reference examples, test examples, and preparation examples, but it goes without saying that the present invention is limited thereto.
reference example 1
[0702] Preparation of 6-methyl-2-(4-trifluoromethylphenyl)nicotinic acid
[0703]
[0704] In the above reaction scheme, Me is methyl, WSC is 1-ethyl-3-(3'-dimethylaminopropyl) carbodiimide, DMAP is dimethylaminopyridine, and DMF is dimethyl formazan amides. Hereinafter, each symbol is the same as defined above.
[0705] a) Methyl 2-chloro-6-methylnicotinate
[0706] 2-Chloro-6-methylnicotinic acid (25.0 g) was suspended in a mixed solvent of dimethylformamide (100 mL) and chloroform (100 mL), and dimethylaminopyridine (21.3 g) and Methanol (4.67 g), and finally, 1-ethyl-3-(3'-dimethylaminopropyl) carbodiimide (WSC) hydrochloride (33.5 g) was added to the mixture, which was then stirred at room temperature After 6 hours, the reaction mixture was concentrated, ethyl acetate (300 mL) was added thereto, the mixture was washed successively with water, 10% ammonium chloride, water, and saturated brine, dried over sodium sulfate, and concentrated, and the residue was subjected...
reference example 2
[0713] Preparation of 3-ethyl-5-fluoro-4-hydroxybenzoic acid methyl ester
[0714]
[0715] In the above reaction scheme, Me is a methyl group; conc.H 2 SO 4 is concentrated sulfuric acid; NBS is N-bromosuccinimide; THF is tetrahydrofuran; MOMCl is chloromethyl methyl ether; nBu is n-butyl; PdCl 2 (PPh 3 ) 2 is dichlorobis(triphenylphosphine)palladium(II); Pd / C is palladium-carbon, and Et is ethyl. Hereinafter, each symbol is the same as defined above.
[0716]a) Methyl 3-fluoro-4-hydroxybenzoate
[0717] To a solution of 3-fluoro-4-hydroxybenzoic acid (3.0 g) in methanol (30 mL) was added concentrated sulfuric acid (3 mL), the mixture was heated to reflux for 5 hours, the reaction solution was left to cool to room temperature, and then concentrated in vacuo. The residue was diluted with ethyl acetate, washed successively with water, saturated aqueous sodium bicarbonate solution, water, and saturated brine, dried over anhydrous sodium sulfate, and concentrated to give...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com