High-dosage extended-release formulation of gepirone
A technology of gepirone and tablets, applied in the field of high-dose delayed-release preparations of gepirone, can solve problems such as adverse side effects, unsatisfactory, and reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0153] A tablet form of the extended release oral dosage form of gepirone was prepared following the process described above and in US Patent No. 5,478,572. Generally, this process requires (i) mixing all of gepirone hydrochloride, all of colorants, all of colloidal silicon dioxide and 20% of hydroxypropyl methylcellulose; (ii) mixing of The mixture of (i) was up to 15 minutes and then crushed using a Fitzmill #0020 plate; (iii) to the blend from (ii) was added all of the microcrystalline cellulose, 50% of magnesium stearate and the remaining 80% of Hydroxypropylmethylcellulose; (iv) blending the blend from (iii) for 28 minutes; (v) slugging the blend from (iv) using a rotary tablet press; (vi) using a Fitzmill #0093 tablet Grinding the pellets from (v); (vii) adding the remaining 50% magnesium stearate to the milled pellets from (vi); (viii) lubricating / blending the mixture from (vii) up to 7 minutes; and (ix) compressing the blend from (viii) into the final desired tablet f...
Embodiment 1
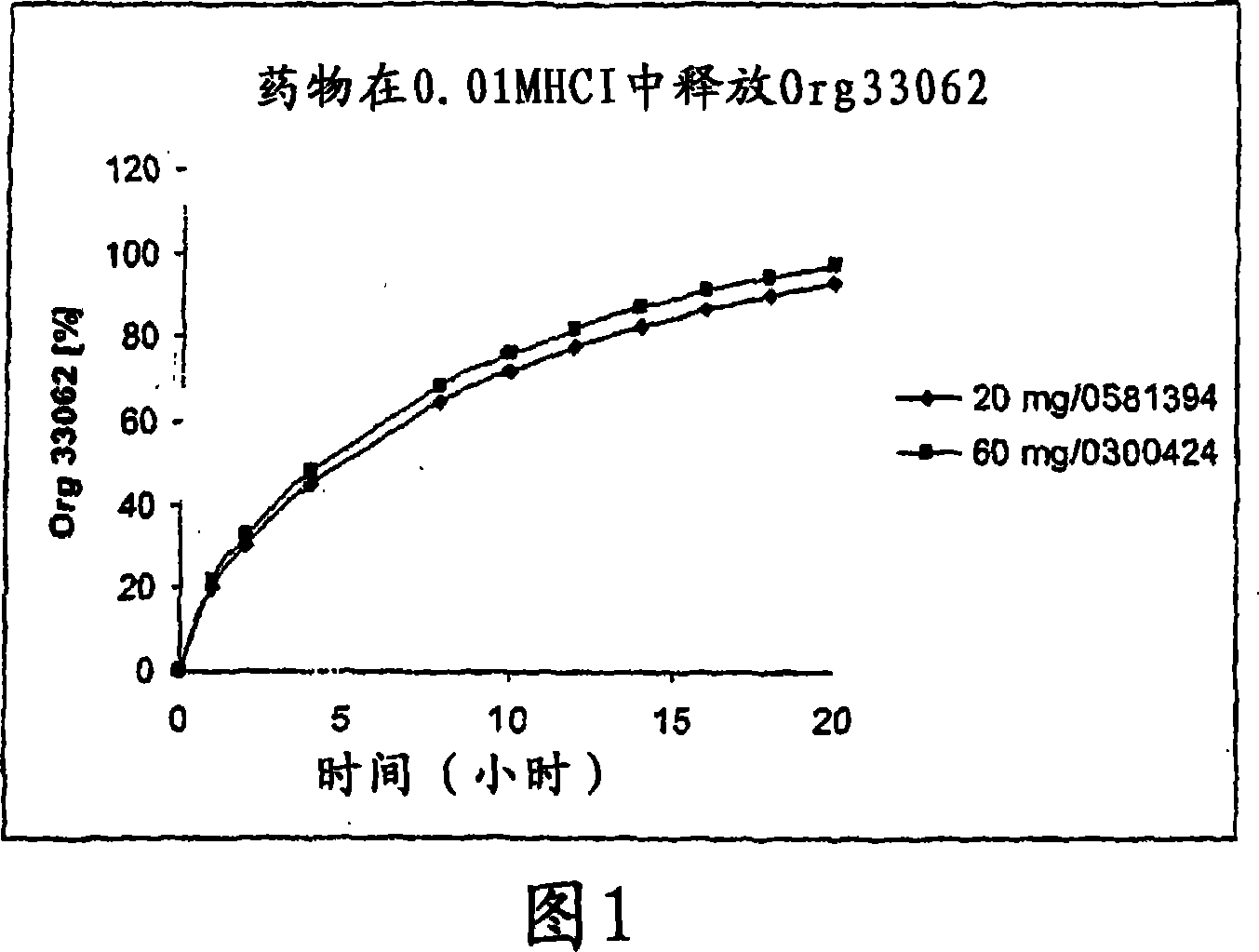
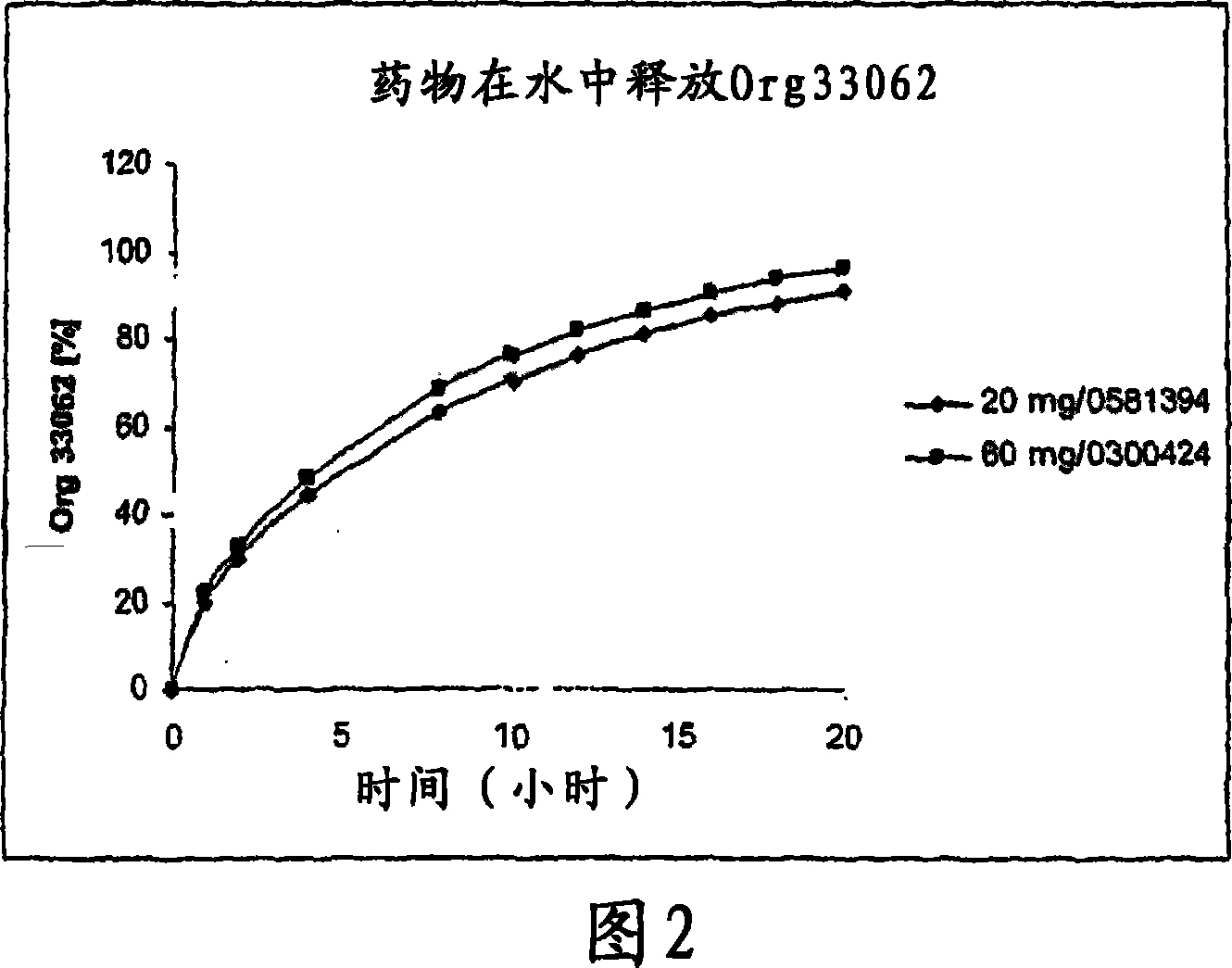
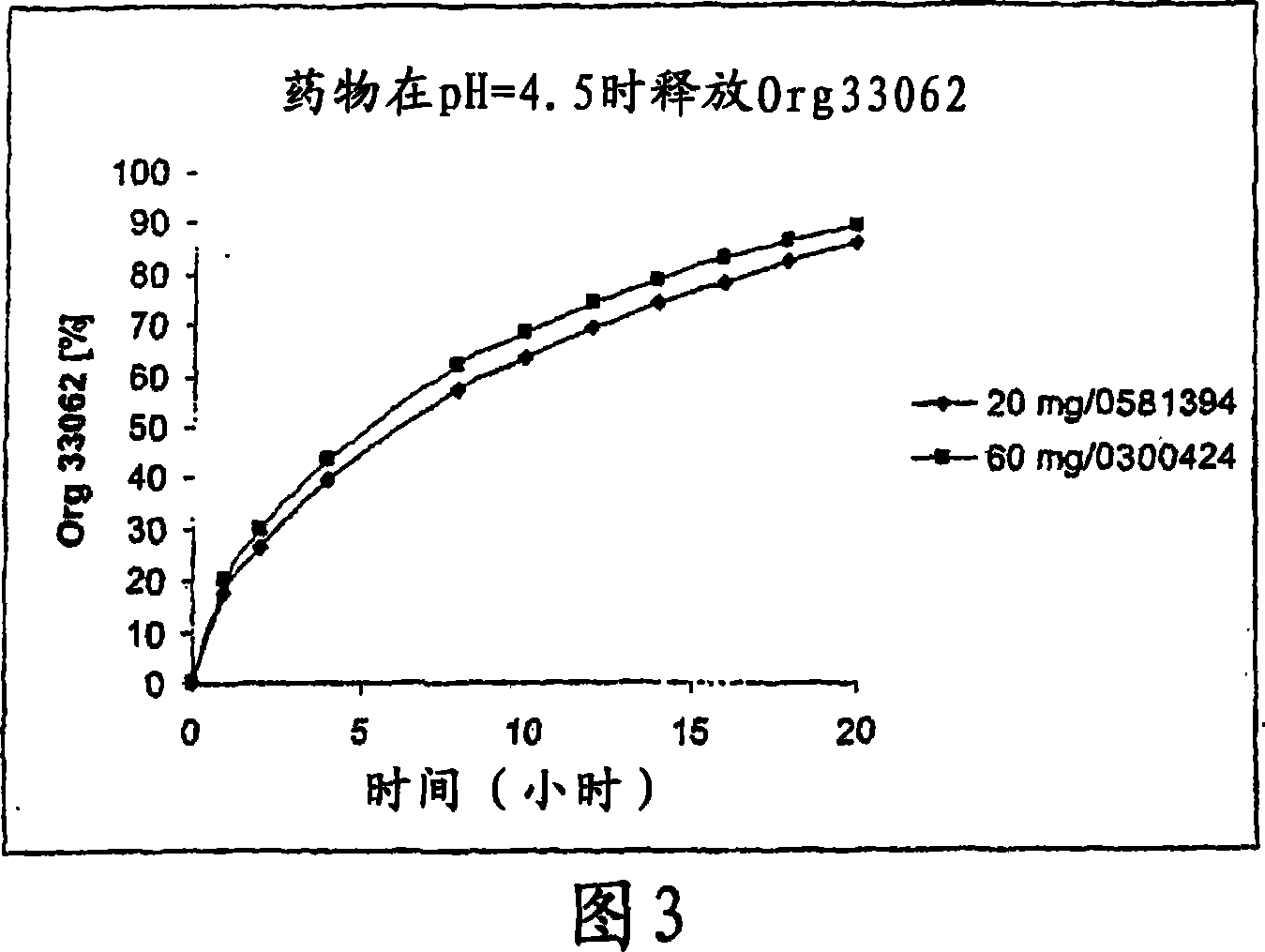
[0157] Example 1: Dissolution mode of 60mg preparation and 20mg preparation
[0158] Table 2 below lists the comparative dissolution profiles of 20 mg and 60 mg tablets in all SUPAC-MR dissolution media.
[0159] time (hours)
medium
intensity
1
2
4
8
10
12
14
16
18
20
0.01M HCl
20mg
20.2
30.4
45.1
64.7
71.8
77.8
82.8
86.9
90.5
93.4
0.01M HCl
60mg
22.0
32.8
48.2
68.6
76.0
82.0
87.1
91.2
94.5
97.3
water
20mg
19.8
29.8
44.0
63.1
70.1
76.0
80.9
85.0
88.4
91.3
water
60mg
21.9
32.7
48.2
68.6
75.8
81.7
86.6
90.5
93.7
96.2
pH=4.5
20mg
17.7
26.6
39.5
57.2
63.8
69.4
74.3
...
Embodiment 2
[0165] Example 2: Bioequivalence of the 80 mg formulation versus the 20 mg formulation
[0166] To date, efficacy and pharmacokinetic data for extended-release gepirone tablets have primarily been obtained by administering one or more 20 mg extended-release gepirone tablets (similar to those described in the preparations above). Therefore, a study was undertaken to demonstrate the bioequivalence of 40 mg (data not shown) with 80 mg extended release gepirone tablets (as described in the preparation above) and multiple 20 mg extended release gepirone tablets.
[0167] To this end, an open-label, randomized, four-way crossover, single-dose study design was employed, with a one-week washout period between treatments. During each study period, subjects were hospitalized from the afternoon until 36 hours after gepirone administration. After excretion, blood samples were collected at 48, 60 and 72 hours post-dose. Thirty-two (32) subjects were administered gepirone during the study...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com