Electrolysing solution acid stripping method and apparatus
A deacidification method and electrolyte technology, applied in circuits, electrical components, secondary batteries, etc., can solve the problems of low deacidification efficiency and resin deacidification efficiency, and achieve the effect of reducing maintenance costs and inhibiting corrosion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
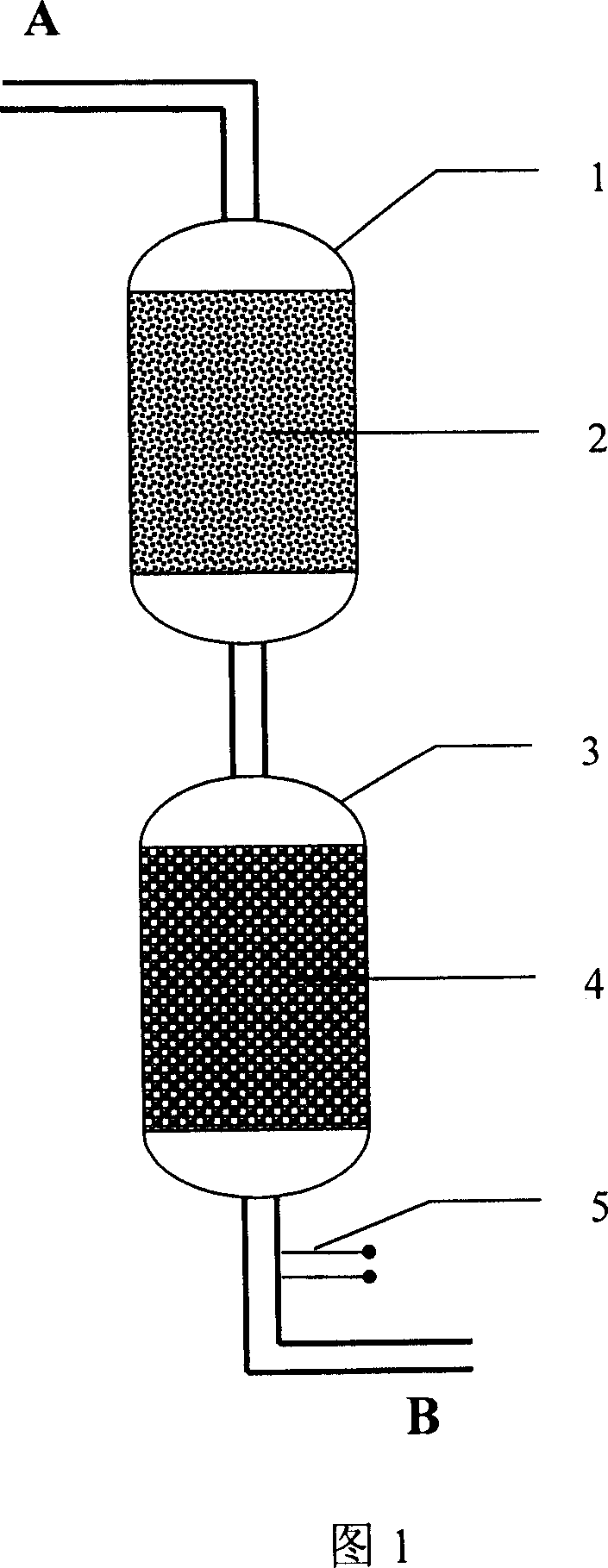
[0042] LiPF 6 Formulated with ethylene carbonate, diethyl carbonate and dimethyl carbonate to make LiPF 6 The electrolyte is 1 mol / liter (the volume ratio of ethylene carbonate, diethyl carbonate and dimethyl carbonate is 1:1:1, and hydrofluoric acid is 120 ppm). The electrolyte passes through the deacidification device shown in Figure 1 at a flow rate of 3 ml / min. In this deacidification device, a weakly basic anion exchange resin container with a diameter of 3 cm is filled with 75 ml of weakly basic anion exchange. Resin (AmberliteIRA-68 with a theoretical adsorption capacity of 1.25 equivalents / liter), a strong alkaline anion exchange resin container with a diameter of 3 cm is filled with 25 ml of strong alkaline anion exchange resin (theoretical adsorption capacity is 1.25 equivalents / liter Amberlite IRA-400), for the adsorption of hydrofluoric acid. After 40 minutes, a sample of the treated electrolyte was taken at the sampling point, and the content of hydrofluoric acid was ...
Embodiment 2
[0044] LiPF 6 Formulated with ethylene carbonate, diethyl carbonate and dimethyl carbonate to make LiPF 6 The electrolyte is 1 mol / L (the volume ratio of ethylene carbonate, diethyl carbonate and dimethyl carbonate is 1:1:1, and hydrofluoric acid is 50 ppm). The electrolyte passes through the deacidification device shown in Figure 1 at a flow rate of 10 ml / min. In this deacidification device, a 3 cm diameter weakly basic anion exchange resin container is filled with 65 ml of weakly basic anion exchange. Resin (Duolite A-30B with a theoretical adsorption capacity of 1.25 equivalents / liter), a strong alkaline anion exchange resin container with a diameter of 3 cm is filled with 35 ml of strong alkaline anion exchange resin (theoretical adsorption capacity is 1.25 equivalents / liter Duolite A162), to carry out the adsorption of hydrofluoric acid. After 40 minutes, a sample of the treated electrolyte was taken at the sampling point, and the content of hydrofluoric acid was analyzed to ...
Embodiment 3
[0046] LiPF 6 Formulated with ethylene carbonate, diethyl carbonate and dimethyl carbonate to make LiPF 6 The electrolyte is 1 mol / L (the volume ratio of ethylene carbonate, diethyl carbonate and dimethyl carbonate is 1:1:1, and hydrofluoric acid is 25 ppm). The electrolyte passes through the deacidification device shown in Figure 1 at a flow rate of 2 ml / min. In this deacidification device, a 3 cm diameter weakly basic anion exchange resin container is filled with 85 ml of weakly basic anion exchange. Resin (DowexMWA1 with a theoretical adsorption capacity of 1.25 equivalents / liter), a strong alkaline anion exchange resin container with a diameter of 3 cm is filled with 15 ml strong alkaline anion exchange resin (theoretical adsorption capacity of Diaion PA412 with a theoretical adsorption capacity of 1.25 equivalents / liter) , Adsorption of hydrofluoric acid. After 40 minutes, a sample of the treated electrolyte was taken at the sampling point, and the content of hydrofluoric aci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com