Loadable polymeric particles for therapeutic and/or diagnostic applications and methods of preparing and using the same
A particle and phosphazene technology, applied in the field of treatment and/or diagnostic use of loadable particles containing polyphosphazene and its preparation and application, can solve the problem of difficulty in suspension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
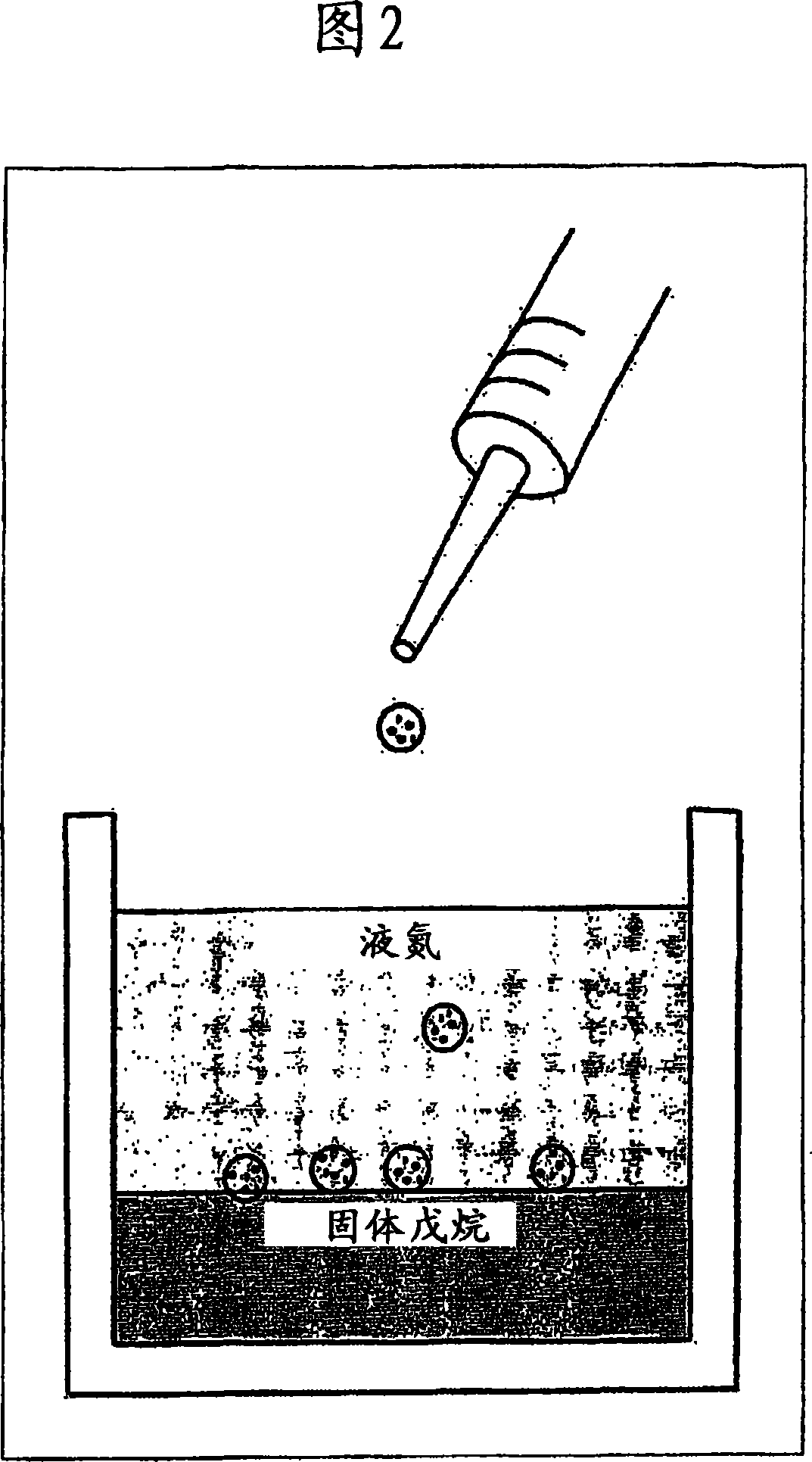
[0080] Microspheres with a diameter of approximately 500 to 600 μm were prepared. First, prepare the polymer solution, including the molecular weight 3 × 10 6 The g / mol of PTFEP polymer was dissolved in the polymer solvent ethyl acetate to obtain a 2% (wt / v) polymer solution. 4 ml of this polymer solution was manually added dropwise into liquid nitrogen using a 5 ml syringe. The dispersion was dispersed on a frozen layer of 150 ml pentane (see Figure 2). A 3-day cryogenic extraction was carried out. Subsequently, the polymer particles were recovered from the reaction tube and air-dried at 21 °C.
Embodiment 2
[0081] Microspheres with a diameter of approximately 350 to 450 μm were prepared. First, prepare the polymer solution, including the molecular weight 3 × 10 6 The g / mol of PTFEP polymer was dissolved in ethyl acetate to obtain a 1% (wt / v) polymer solution. 4 ml of this polymer solution was manually added dropwise into liquid nitrogen using a 5 ml syringe. The dispersion was dispersed on a frozen layer of 150 ml pentane (see Figure 2). A 3-day cryogenic extraction was carried out. Subsequently, the polymer particles were recovered from the reaction tube and air-dried at 21 °C.
Embodiment 3
[0082]Microspheres with a diameter of approximately 500 to 600 μm were prepared. First, prepare the polymer solution, including the molecular weight 12 × 10 6 The g / mol of PTFEP polymer was dissolved in methyl isobutyl ketone to obtain a 2% (wt / v) polymer solution. 4 ml of this polymer solution was manually added dropwise into liquid nitrogen using a 5 ml syringe. The dispersion was dispersed on a frozen layer of 150 ml of a 1:9 (v / v) mixture of ethanol / pentane (see Figure 2). A 3-day cryogenic extraction was carried out. Subsequently, the polymer particles were recovered from the reaction tube and dried under low pressure at 21°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com