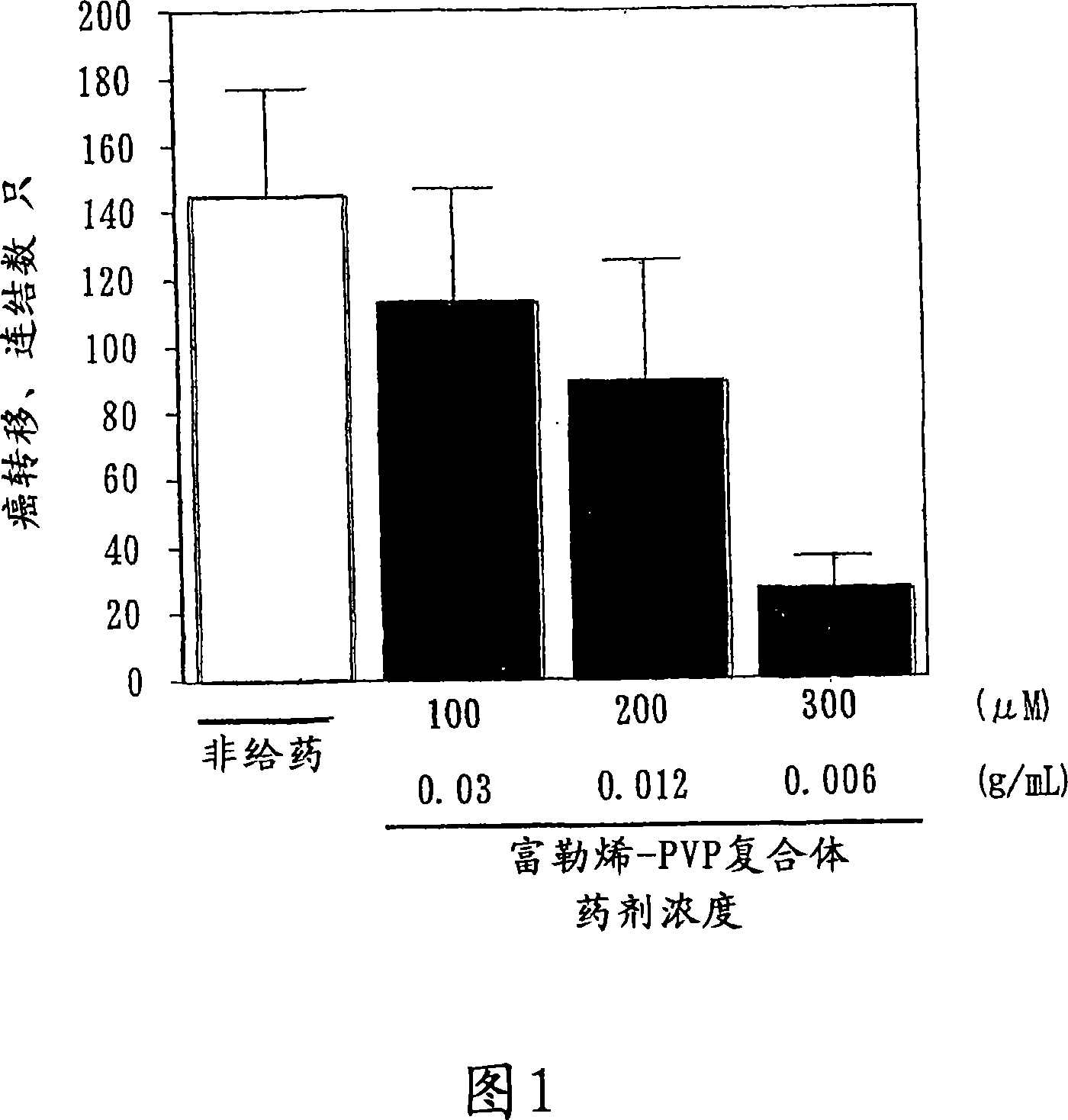
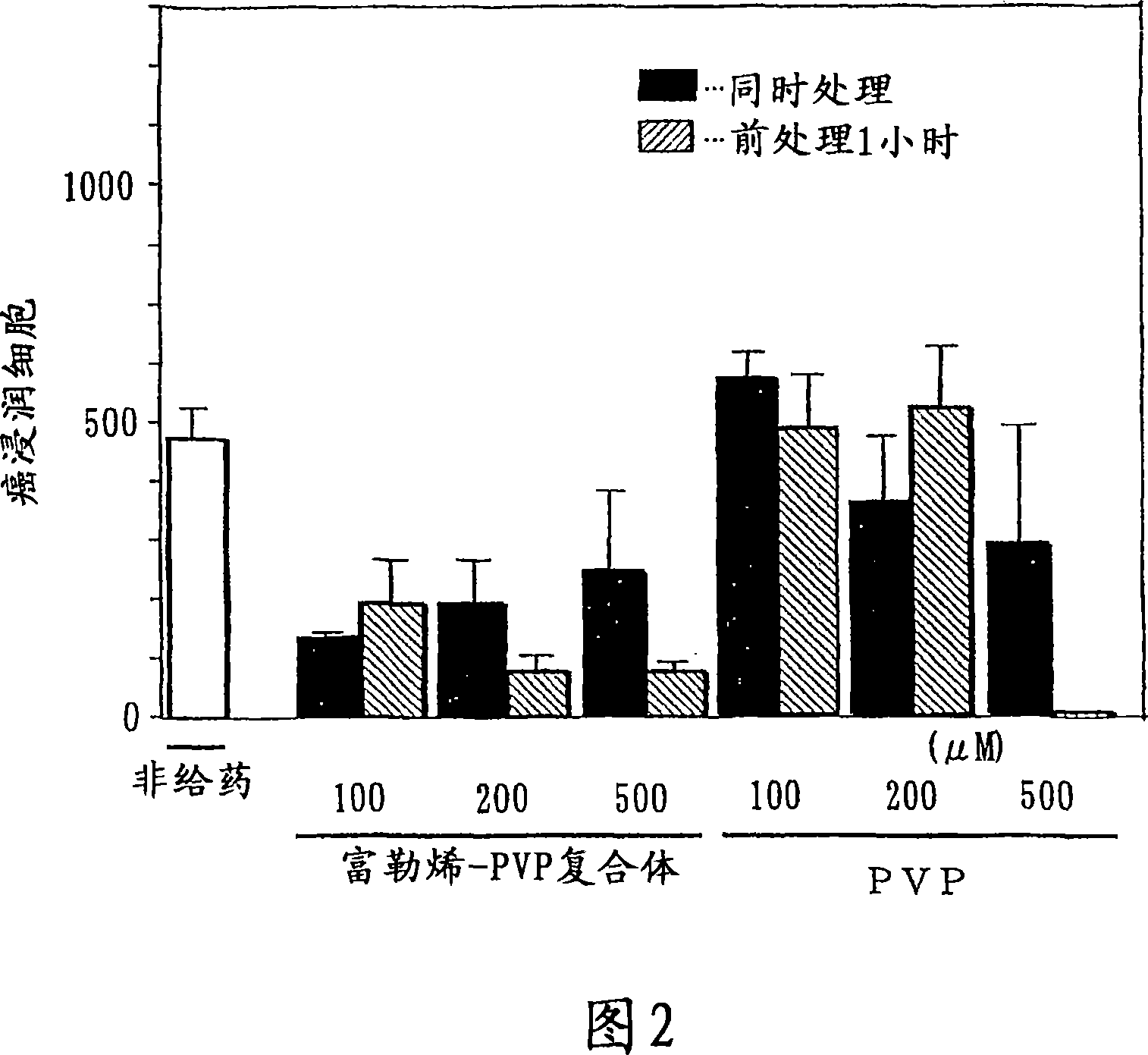
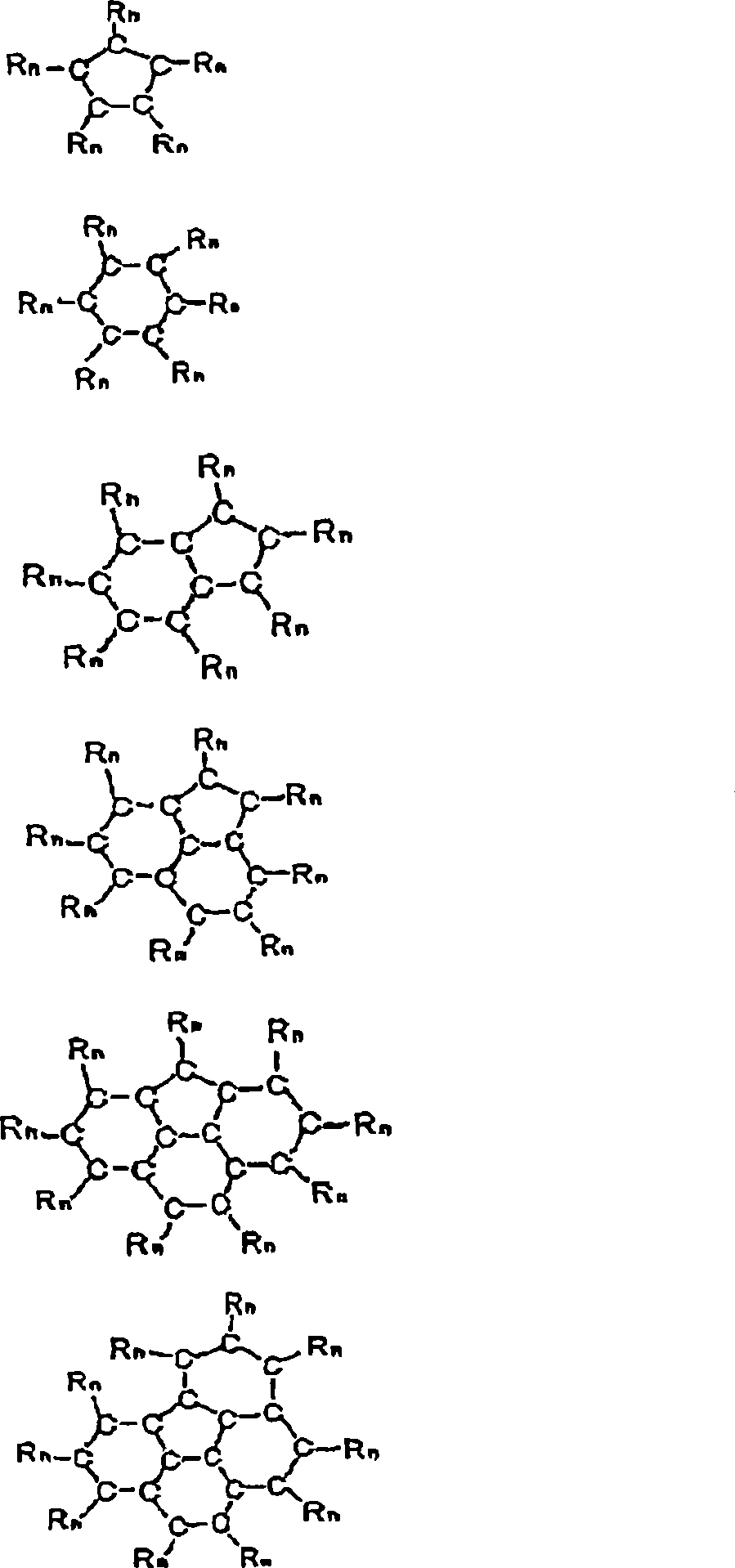
Preventive/therapeutic composition for free radical disease
A disease prevention and disease treatment technology, applied in the direction of non-active ingredients of polymer compounds, antiviral agents, drug delivery, etc., can solve the problems of easy disappearance of activity, inability to fully exert free radical elimination, instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0188] Embodiment 1: injection solution A
[0189] The mixture of cyclodextrin inclusion C60 fullerene and cyclodextrin inclusion C70 fullerene uniformly mixed in a weight ratio of 8:2 is completely dissolved in 1000cc of pharmaceutical Ringer's solution at a concentration of 178ppm. Sterile filtration is performed with a sterilizing filter for the production of injection solution, and an injection solution is produced as the drug for preventing and treating free radical diseases for intravenous injection of the present invention.
Embodiment 2
[0190] Embodiment 2: injection solution B
[0191] A mixture in which hydroxylated C60 fullerene with a hydroxylation rate of 87% (mole conversion) and hydroxylated C70 fullerene with a hydroxylation rate of 61% (mole conversion) was uniformly mixed in a weight ratio of 8:2, at 5ppm After being completely dissolved in 1000 cc of Ringer's solution for medicine, the injection solution was sterilized and filtered with a sterilizing filter for the manufacture of an injection to prepare an injection solution as the drug for the prevention and treatment of free radical diseases for intravenous injection of the present invention.
Embodiment 3
[0192] Example 3: Injection Solution C
[0193]Fullerenes manufactured by Bitamin C60 Biorisaichi Co., Ltd. under the trade name Radical Ponji (fullerene concentration: 200 ppm) were completely dissolved in 1000 cc of Ringer's solution for medicine at a concentration of 1%, and then used for the production of injections. Bacteria filter is carried out sterilizing filtration, and injection solution is manufactured as the preventive and therapeutic drug for free radical disease for intravenous injection of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com