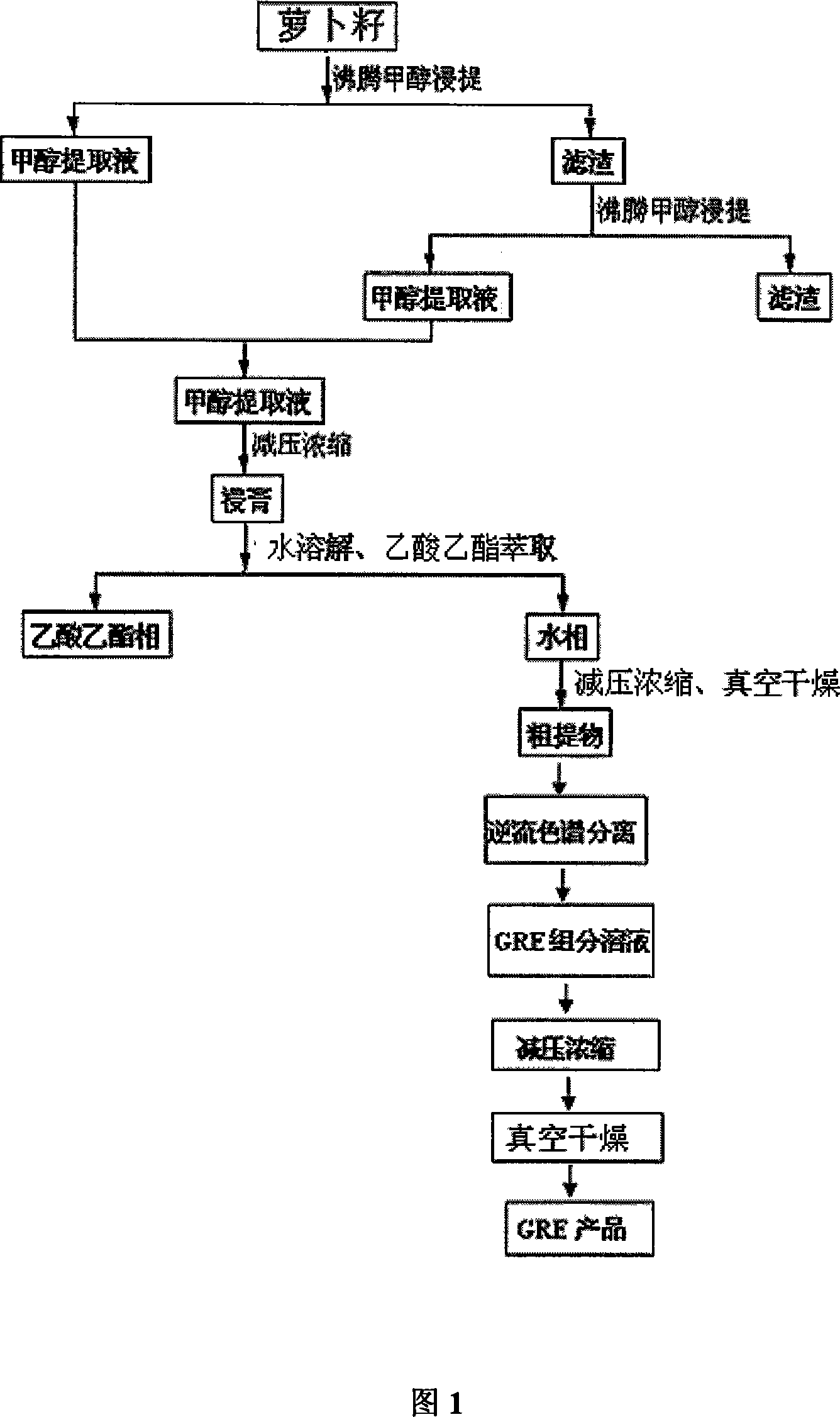
Method for separating 4-methylsulphinyl-3-cyclobutenyl glucosinolates
A technology of methylsulfinyl and butenyl sulfide, applied in the field of food processing, can solve the problems of less separation, high cost of glucosinolates, large separation technology, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
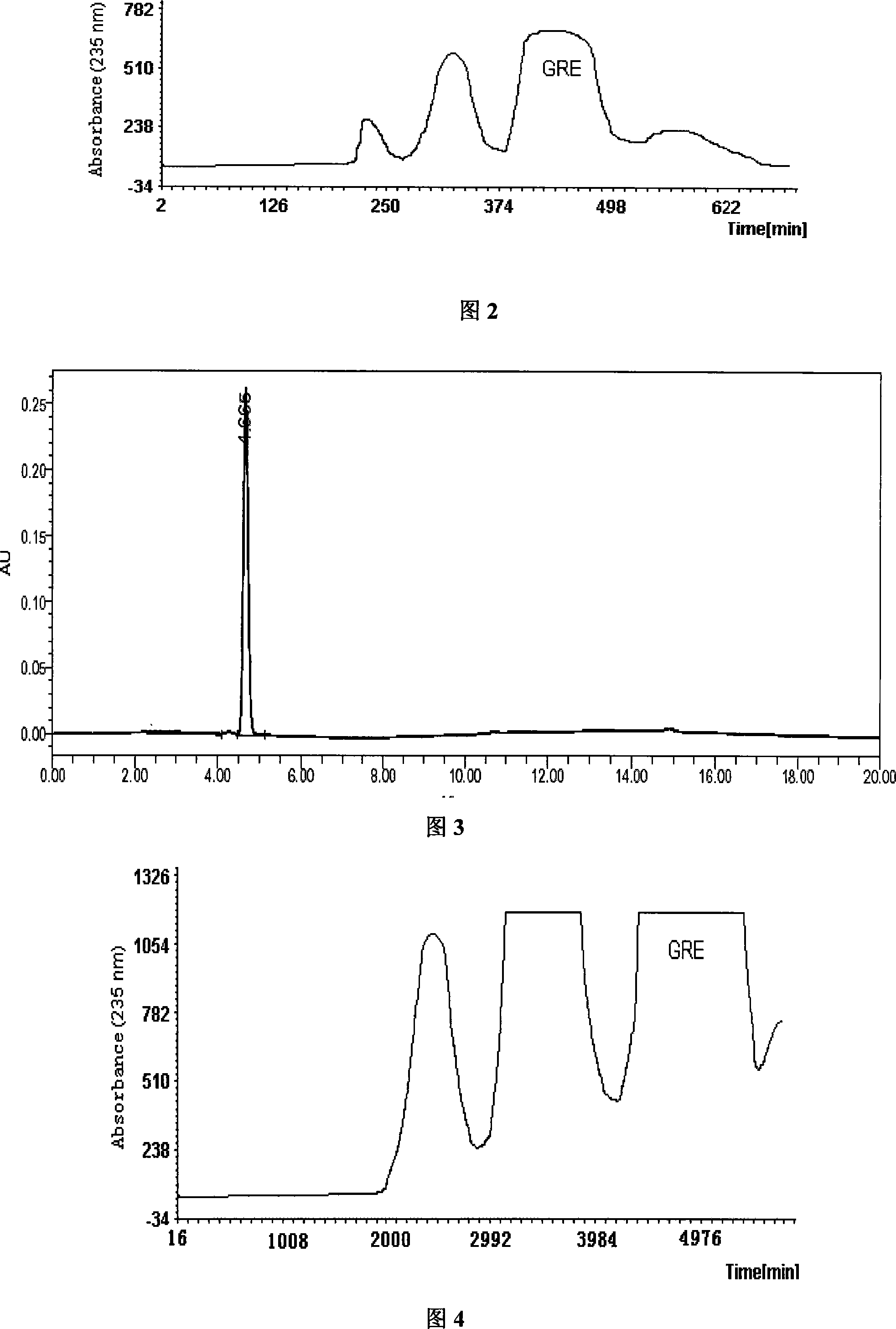
[0022] The solvent system of the present embodiment adopts n-butanol-acetonitrile-5% ammonium sulfate solution (10:5:4); The countercurrent chromatograph is HSCCC-D1000 high-speed countercurrent chromatograph (column capacity 1L), and GRE in the crude extract of GRE is about 15%.
[0023] Measure 2000ml of n-butanol, 1000ml of acetonitrile, and 800ml of 5% ammonium sulfate solution, put it in a 5L separatory funnel, shake it well, and put the upper and lower phases into reagent bottles after standing for stratification. The lower phase was injected into the chromatographic column of high-speed countercurrent chromatography at a flow rate of 10 ml / min. Weigh 2.0 g of the GRE crude extract and dissolve it in 50 ml upper phase to prepare a countercurrent chromatography sample solution, turn on the countercurrent chromatograph to 800 rpm, and then input the sample solution at a flow rate of 1.0ml / min. The flow rate of 2.0ml / min was input into the mobile phase to elute the compone...
Embodiment 2
[0025] The solvent system of the present embodiment adopts n-butanol-acetonitrile-5% ammonium sulfate solution (10:3:4); About 15%.
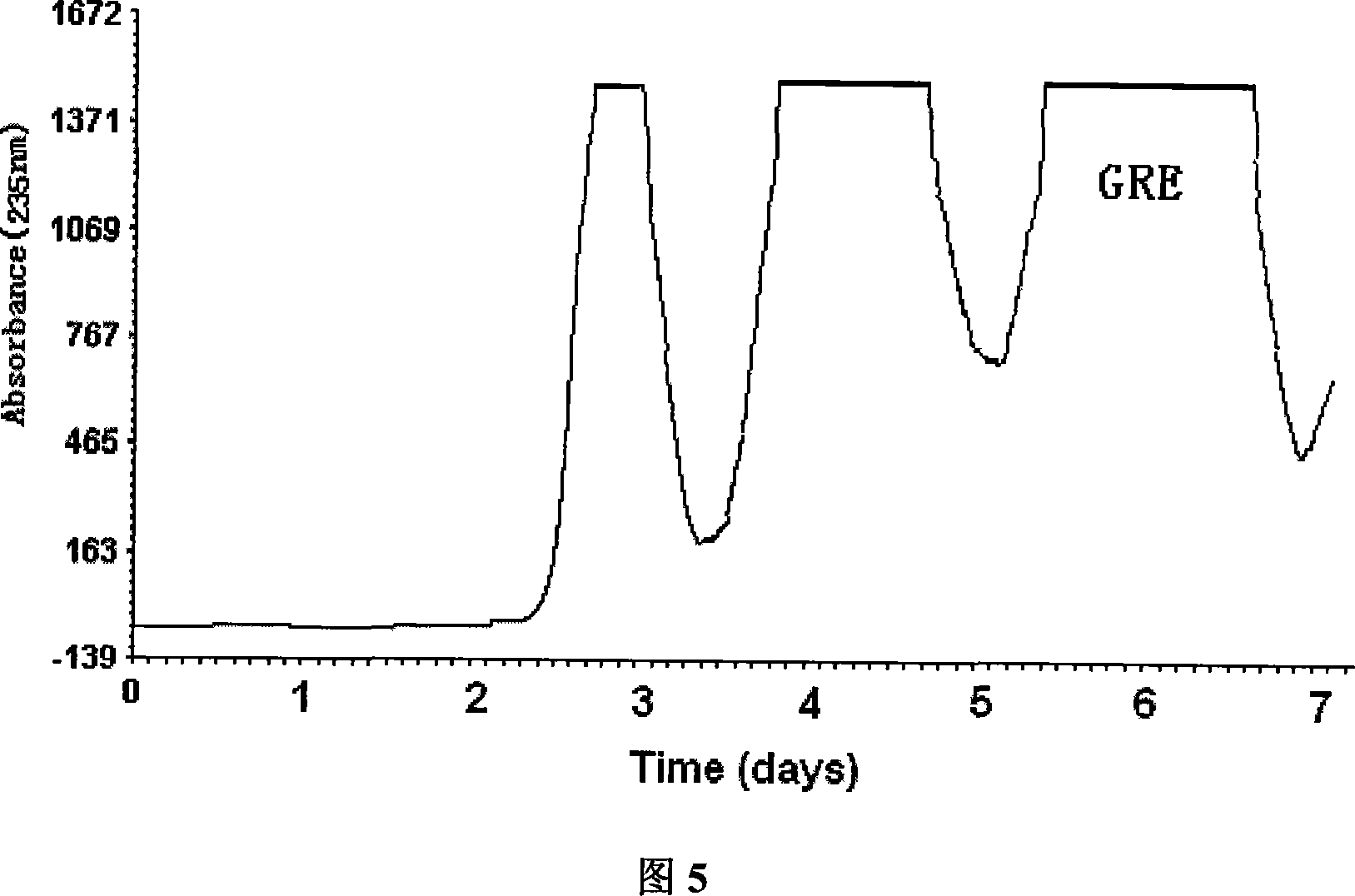
[0026] Measure 8L of n-butanol, 2.7L of acetonitrile, and 3.2L of 5% ammonium sulfate solution, put them in a 20L separatory bottle, mix thoroughly, and after standing for stratification, put the upper and lower phases into the reagent bottle respectively. Inject the lower phase into the chromatographic column of the low-speed countercurrent chromatography at a flow rate of 20ml / min until the chromatographic column is completely filled. Weigh 30.0g of the GRE crude extract and dissolve it in 500ml upper phase to prepare a countercurrent chromatography sample solution, turn on the countercurrent chromatograph to 50 rpm, and then input the sample solution at a flow rate of 2.0ml / min. The flow rate of 5.0ml / min was input into the mobile phase to elute the components in the column, and the countercurrent chromatographic effluent was detected at 235...
Embodiment 3
[0028] The solvent system of the present embodiment adopts n-butanol-acetonitrile-5% ammonium sulfate solution (10:6:4); The countercurrent chromatograph is RSCCC-D40000 low-speed countercurrent chromatograph (column capacity 40L), and GRE in the crude extract of GRE is about 15%.
[0029] Measure 80L of n-butanol, 48L of acetonitrile, and 32L of 5% ammonium sulfate solution, place them in a 200L separatory tank, mix thoroughly, and after standing for stratification, put the upper and lower phases into reagent bottles respectively. The lower phase is injected into the chromatographic column of the low-speed countercurrent chromatography at a flow rate of 200ml / min until the chromatographic column is completely filled. Weigh 300.0g of the GRE crude extract and dissolve it in 5000ml upper phase to prepare a countercurrent chromatography sample solution, turn on the countercurrent chromatograph to 50 rpm, and then input the sample solution at a flow rate of 10.0ml / min. The flow ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com