Staphylokinase intramuscular injection and method for preparing the same
A technology of staphylokinase and injection, which is applied in the field of intramuscular injection of thrombolytic drugs and its preparation, and can solve problems such as hindering the repeated use of Sak
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
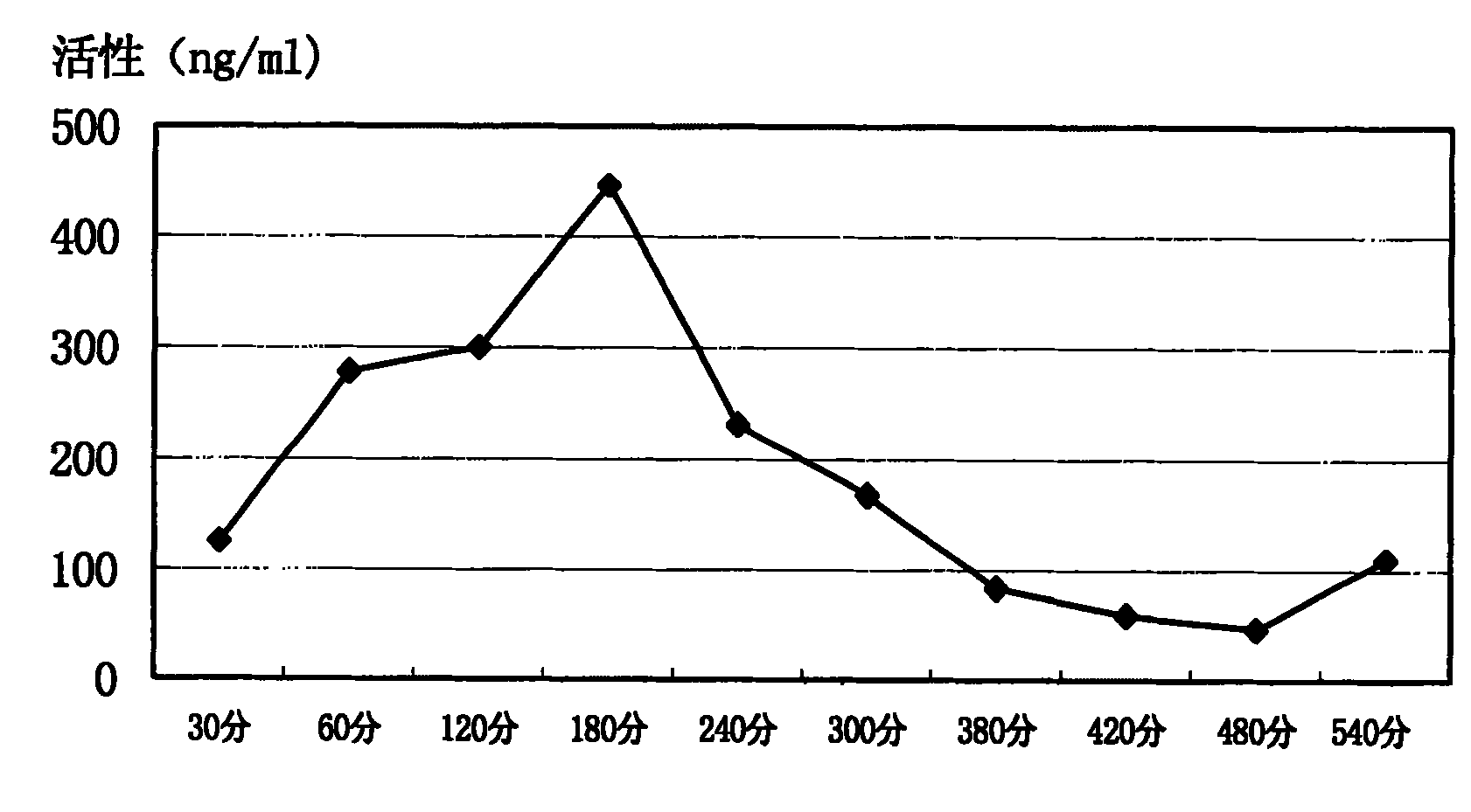
[0087] A kind of preparation method of preferred staphylokinase intramuscular injection comprises the steps: appropriate amount of staphylokinase and phosphate are dissolved in distilled water, make the staphylokinase intramuscular injection containing the phosphate of 0.5-40mg / ml staphylokinase and 3-15mM , adjust the pH to a suitable range (such as 6.5-7.5), then divide into suitable containers (such as 1ml, 2ml, 5ml or 10ml ampoules, etc.), and freeze-dry.
[0088] The active ingredient staphylokinase that can be used in the staphylokinase intramuscular injection of the present invention can be natural staphylokinase or recombinant staphylokinase, preferably recombinant staphylokinase; Described recombinant staphylokinase includes various mutants produced by gene mutation, and PEGylated Various derivatives of staphylokinase. Especially preferred are low antigenicity staphylokinase mutants.
[0089] A preferred method for preparing staphylokinase intramuscular injection com...
Embodiment 1
[0100] Preparation of Recombinant Staphylokinase with Low Antigenicity
[0101] The results of bioinformatics analysis of staphylokinase showed that Sak contains three non-overlapping antigenic determinants, and the specific positions of two antigenic determinants have been elucidated. In this example, the deletion of the staphylokinase gene leads to changes in its spatial structure, thereby affecting the antigenicity of the glucosylase, and obtaining a low-antigenic staphylokinase.
[0102] Now take the deletion of 21 amino acids at the N-terminal of natural staphylokinase as an example.
[0103] Construction of recombinant staphylokinase gene
[0104] Primers were designed as follows:
[0105] P15’-CAC GAA TTC ATG GGC CCG TAT TTG ATG GTA-3’
[0106] P25’-CAC GGA TCC TTA TTT CTT TTC ATA AAC AAC CTT-3’
[0107] Obtain the mutant gene by PCR, and the reaction steps of PCR are:
[0108] After adding DNA template, buffer, dNTP, primers P1 and P2, make up 50 microliters with ...
Embodiment 2-3
[0122] Preparation of Recombinant Staphylokinase with Low Antigenicity
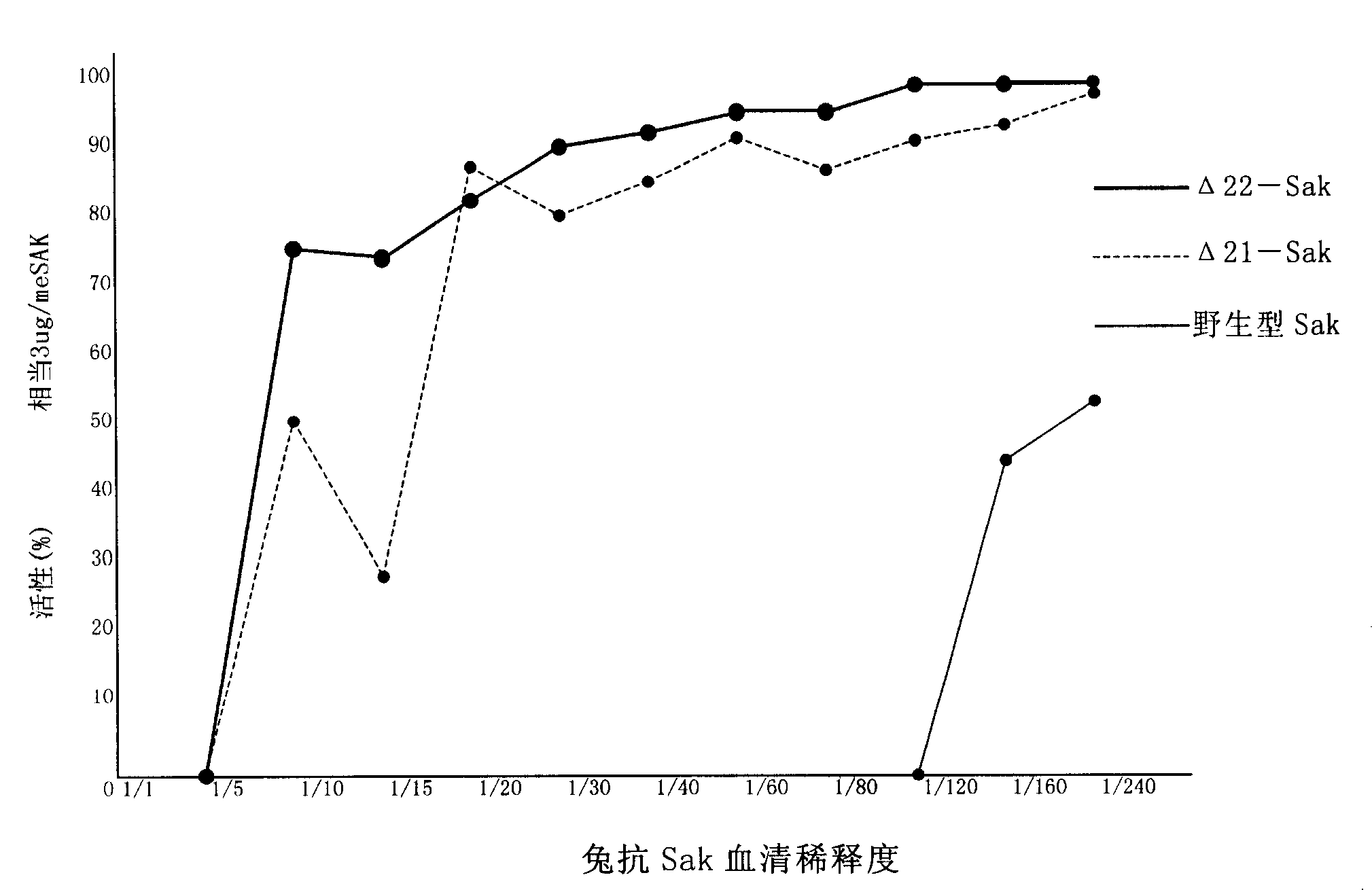
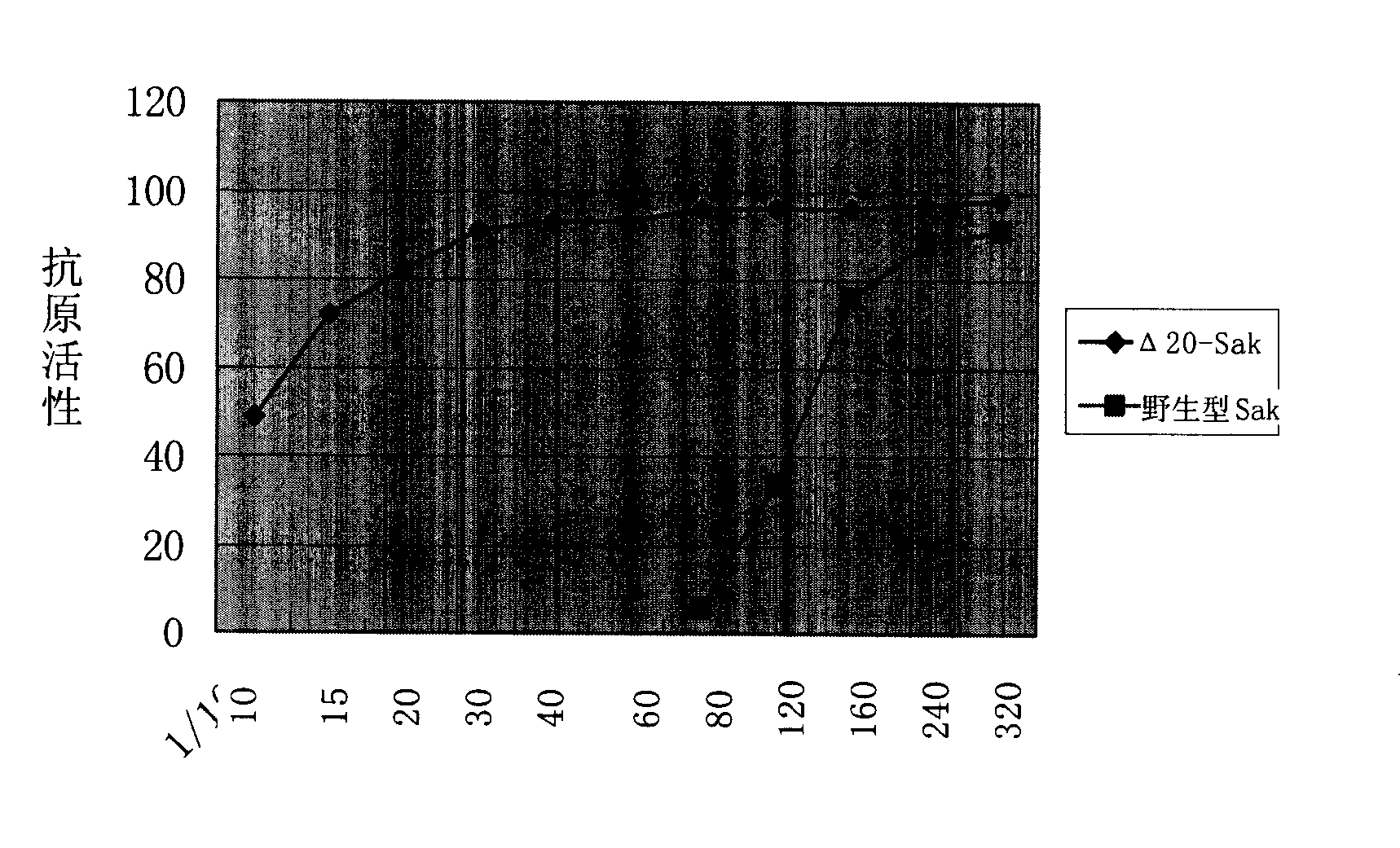
[0123] A method similar to Example 1 was used to obtain low antigenic staphylokinase that lacked 20 and 22 amino acids at the N-terminal of natural staphylokinase, and the mutant genes were respectively Δ20-Sak (SEQ ID NO: 1) and Δ22-Sak (SEQ ID NO :3); its amino acid sequences are respectively SEQ ID NO: 4 (116 amino acids, molecular weight about 13200 Daltons) and SEQ ID NO: 6 (114 amino acids, molecular weight about 13000 Daltons).
[0124] The determination of low antigenicity was carried out separately, and the results are shown in figure 2 and image 3 .
[0125] The results showed that the staphylokinase obtained from the mutant strain Δ20 and the mutant strain Δ22 had the same low antigenicity as the staphylokinase obtained from the mutant strain Δ21.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com