Application of HSP27 in aspect for improving post-ischemic cardiac systolic function
A technology for heart injury and use, applied in the field of biotechnology or medicine, can solve problems affecting the contraction function of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
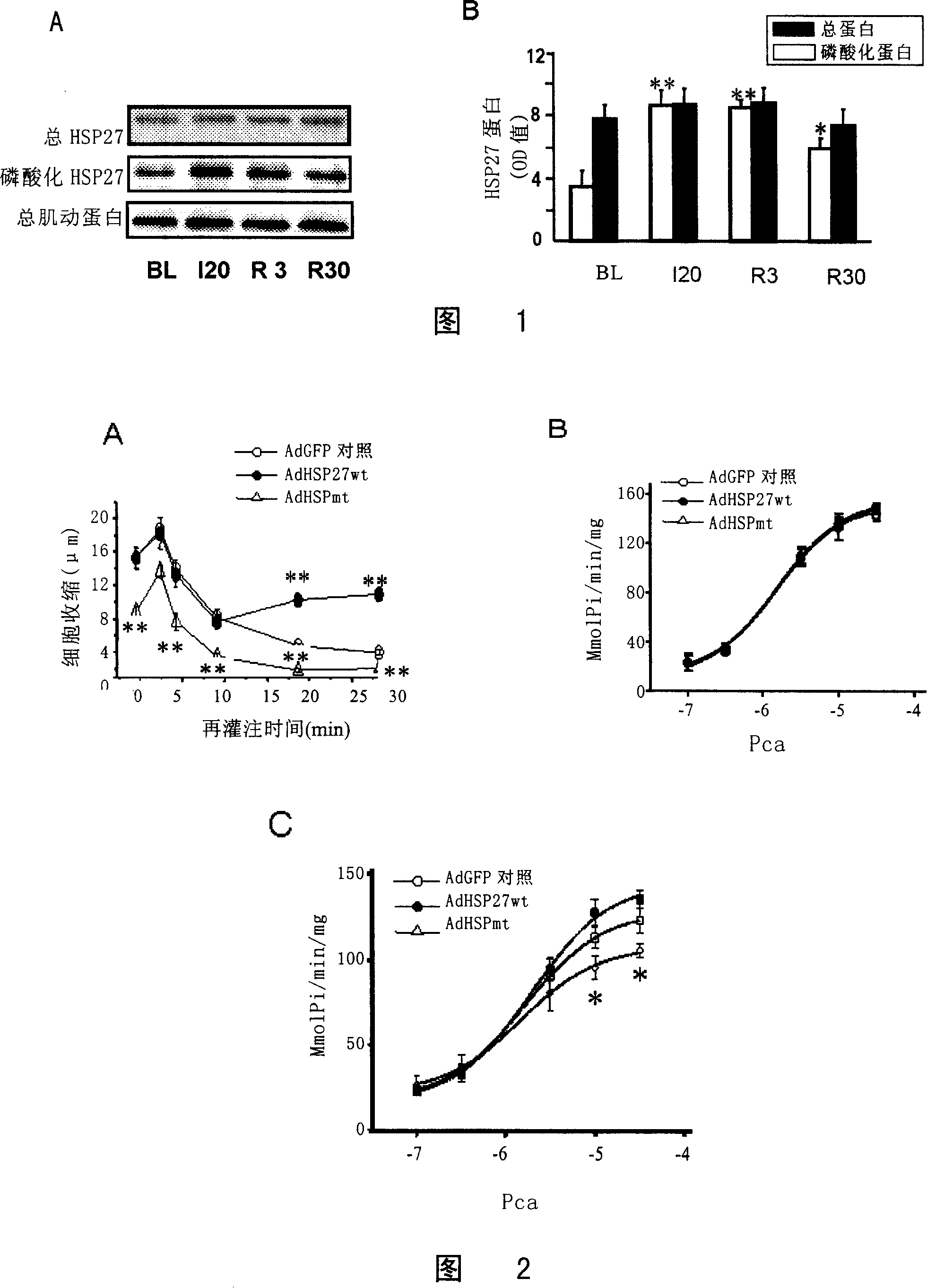
[0162] Example 1 Expression of HSP27 in ischemia / reperfusion cardiomyocytes
[0163] In this embodiment, the expression of HSP27 in ischemia / reperfusion cardiomyocytes was determined by Western Blot test.
[0164] The results of Western Blot are shown in Figure 1A and Figure 1B. The results showed that there was no significant difference in the expression of HSP27 total protein at 20 minutes of ischemia, 3 minutes and 30 minutes of reperfusion compared with the balance period, while the expression of phosphorylated protein was significantly different. Significantly increased (P<0.01).
Embodiment 2
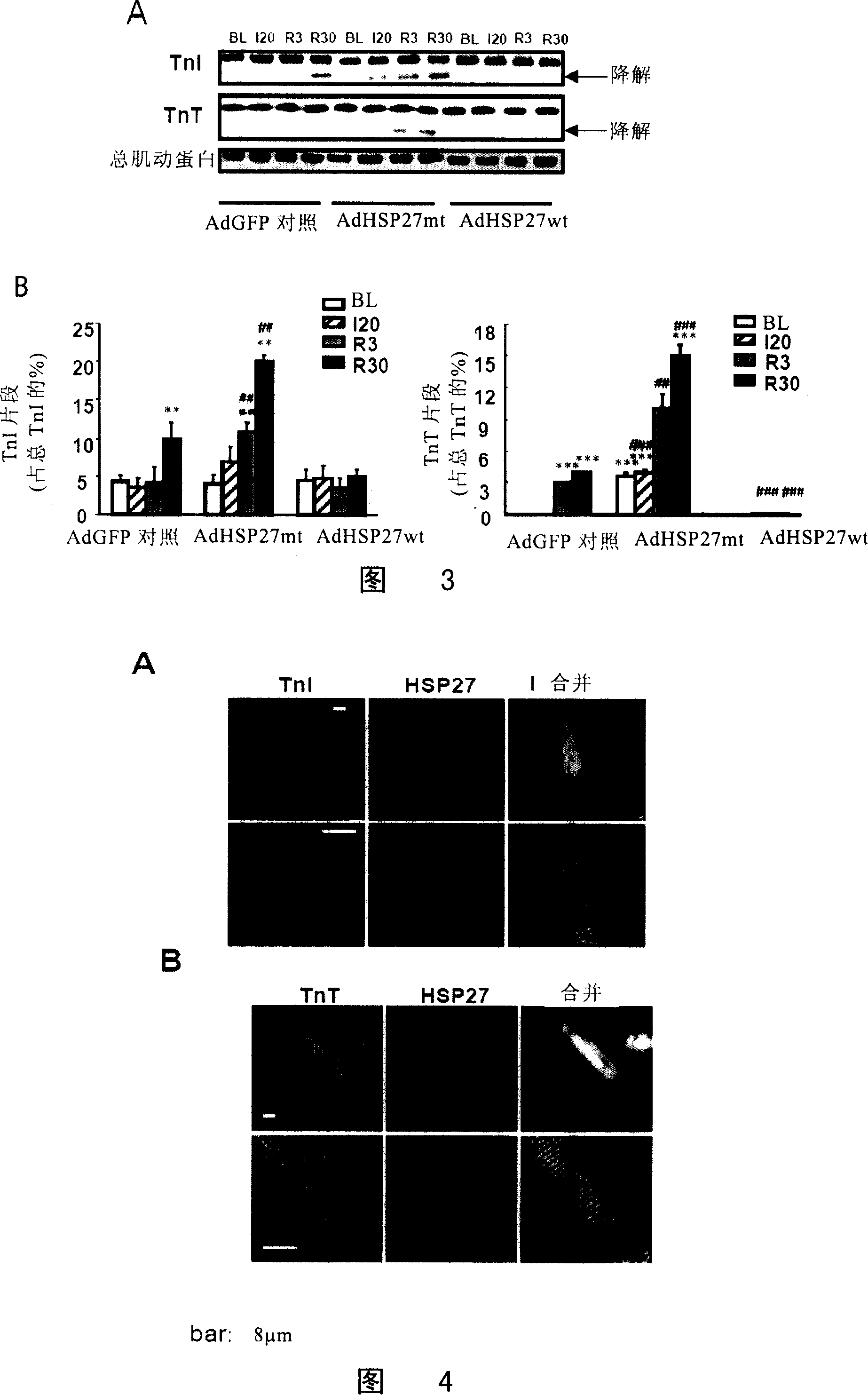
[0166] Overexpression of wild-type hsp27 gene can improve the contractility of cardiomyocytes after ischemia / reperfusion
[0167] In order to determine whether HSP27 is related to myocardial contraction, the inventors first detected its effect on contractile function at the level of cardiomyocytes.
[0168] The carriers used in the experiment were divided into the following three groups:
[0169] (1) Transfection of cardiomyocytes with control empty vector AdGFP (i.e. control group (CON));
[0170] (2) AdHSP27wt vector expressing wild-type hsp27 gene; and
[0171] (3) AdHSP27mt vector expressing mutant hsp27 gene.
[0172] The results showed that during the 30-minute reperfusion period after 20 minutes of simulated ischemia, the cell contraction amplitude of the AdGFP group was significantly lower than that before ischemia, while the cell contraction amplitude of the AdHSP27wt group was significantly stronger than that of the control group at the late stage of reperfusion g...
Embodiment 3
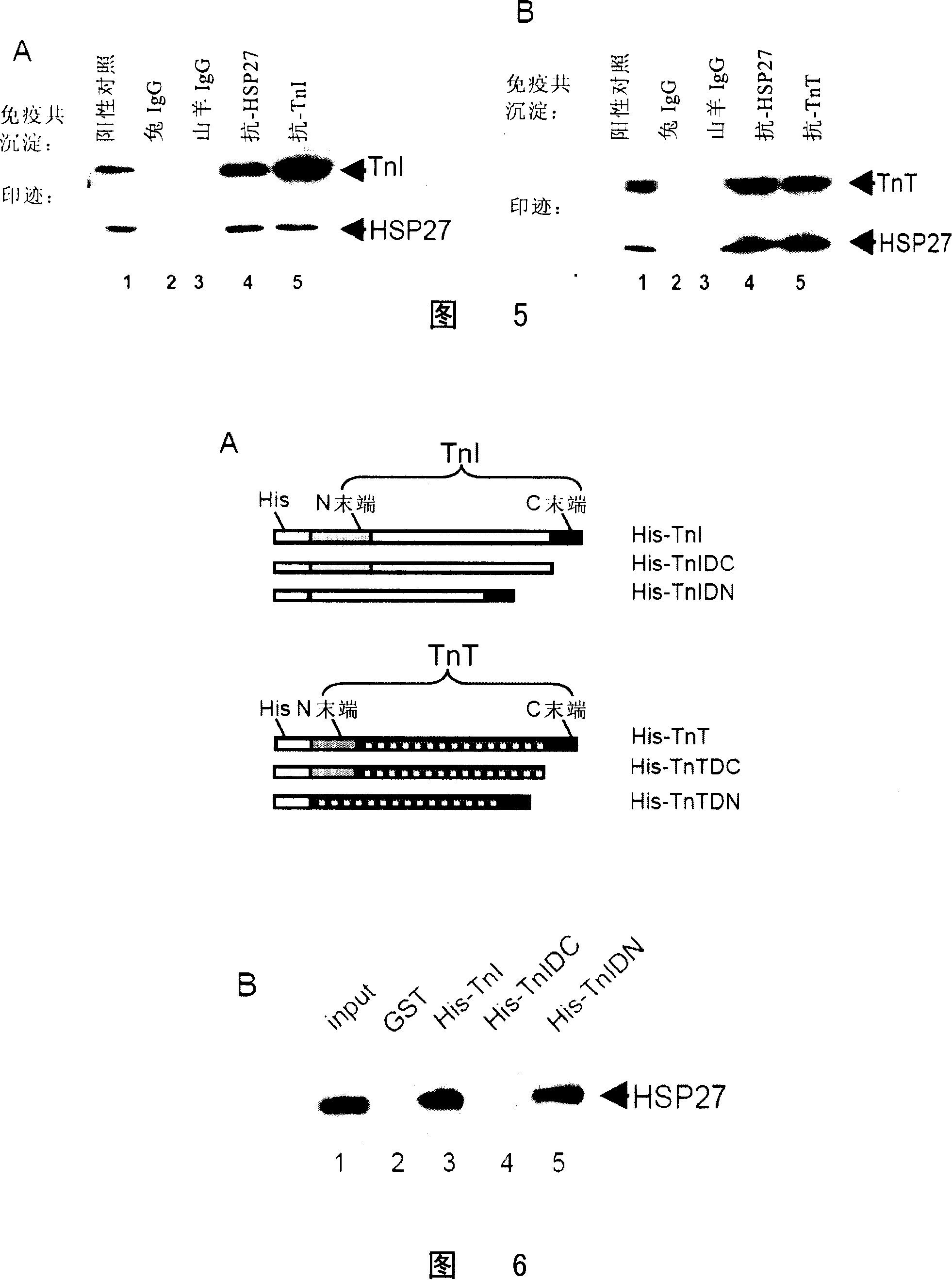
[0175] Example 3 Effect of HSP27 on TnI and TnT Degradation Caused by Ischemia / Reperfusion
[0176] In search of the target of HSP27 on myofilament, the inventors found that ischemia / reperfusion injury can cause the degradation of cardiomyocyte TnI and TnT.
[0177] The results of Western Blot showed that in the cells transfected with AdHSP27wt group, the degradation of TnI and TnT small molecular protein was significantly lower than that in the control group (P<0.01); while the degradation degree of TnI and TnT in the cells transfected with AdHSP27mt group was lower than that of the control group. Significantly higher than the control group (P <0.01, Figure 3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com