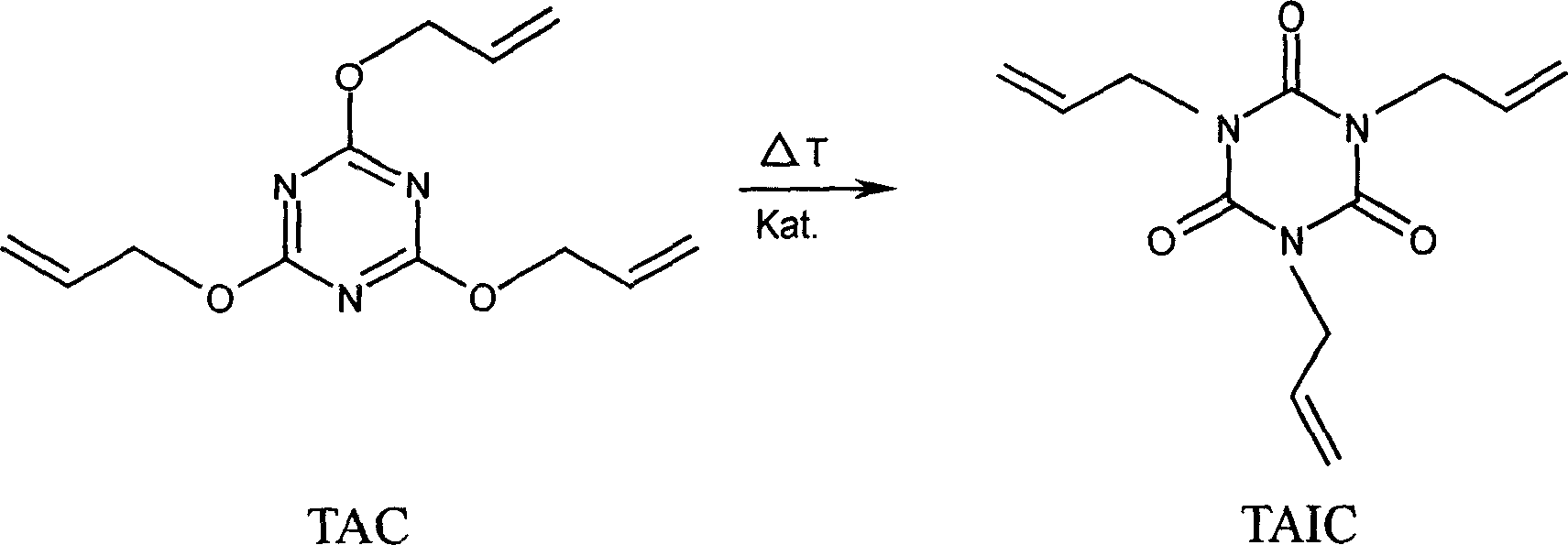
Method for preparing triallyl isocyanurate
A technology of triallyl isocyanurate and triallyl cyanurate, which is applied in the field of manufacturing triallyl isocyanurate, can solve the problems of promoting the formation of by-products and insufficient conversion rate, so as to avoid Aggregation, potential hazard minimization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] In a 500-ml capacity double-wall stirred vessel heated with a constant temperature polysiloxane bath adjusted to 130°C, add 25 ml of TAC and 0.3 g of CuCl 2 (without water) in 75 ml of toluene. The temperature inside the flask was adjusted to 113 to 115°C. After 16 minutes, vigorous reflux began and the initially blue solution turned dark gray-green. After the reaction gradually weakened, a metering pump was used to continuously pump 3000 ml of TAC, 3.0 g of CuCl at 10 ml / min. 2 and 3000 ml of toluene. Simultaneously, 10 ml / min of the reaction solution was pumped out using a second metering pump. The reaction temperature rises to about 123 to 125°C.
[0045] If working with an apparatus with a higher TAC concentration (higher space-time yield), the reaction temperature is limited to a maximum of 140° C. by applying a corresponding vacuum.
[0046] The conversion rate is greater than 99.9%. Diallyl isocyanurate is formed as a by-product by TAC and residual water in...
Embodiment 2
[0048] According to the method of Example 1, but using diethyl carbonate as solvent. By mixing TAC with diethyl carbonate at a volume ratio of 1:3, and adding 2.5 g of CuCl 2 / liter of the starting reactant solution and then very finely ground in a wet mill to make the reaction mixture for the starting reaction. Preload 200 ml of the solution, heat to 130 °C and wait for the reaction to start, then pump TAC and diethyl carbonate at a volume ratio of 1:1 and 0.4 g of CuCl at 15 ml / min. 2 / liter of solution composed of a homogeneous dispersion while pumping out 15 ml / min of green TAIC solution. The conversion of TAC was greater than 99.8%. The process step can be maintained without hindrance for a period of 8 hours without loss of yield and purity.
Embodiment 3
[0050] According to the method of Example 1, but after starting the reaction, metered in 1000 ml of TAC and 0.25 g of anhydrous CuCl 2 A very finely dispersed homogeneous mixture of compositions. The metered amount was 10 ml / min; at the same time 10 ml / min of the reaction solution was pumped out. As the amount of toluene in the reaction vessel decreases over time by distilling off the toluene, the operating temperature in the reactor continues to rise. To prevent polymerization, a vacuum was applied at an internal temperature of the flask of 140°C, thereby obtaining a constant working temperature by vapor cooling the TAIC formed. The vacuum required here is 2.0 to 3.0 hPa. The method provides TAIC with a purity of 98.5%; the isomerization rate of TAIC is greater than 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com