Method for synthesizing target compound-ligustrazine derivant preventing cerebrovascular disease
A technology for cardiovascular and cerebrovascular diseases and compounds, applied in 2 fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
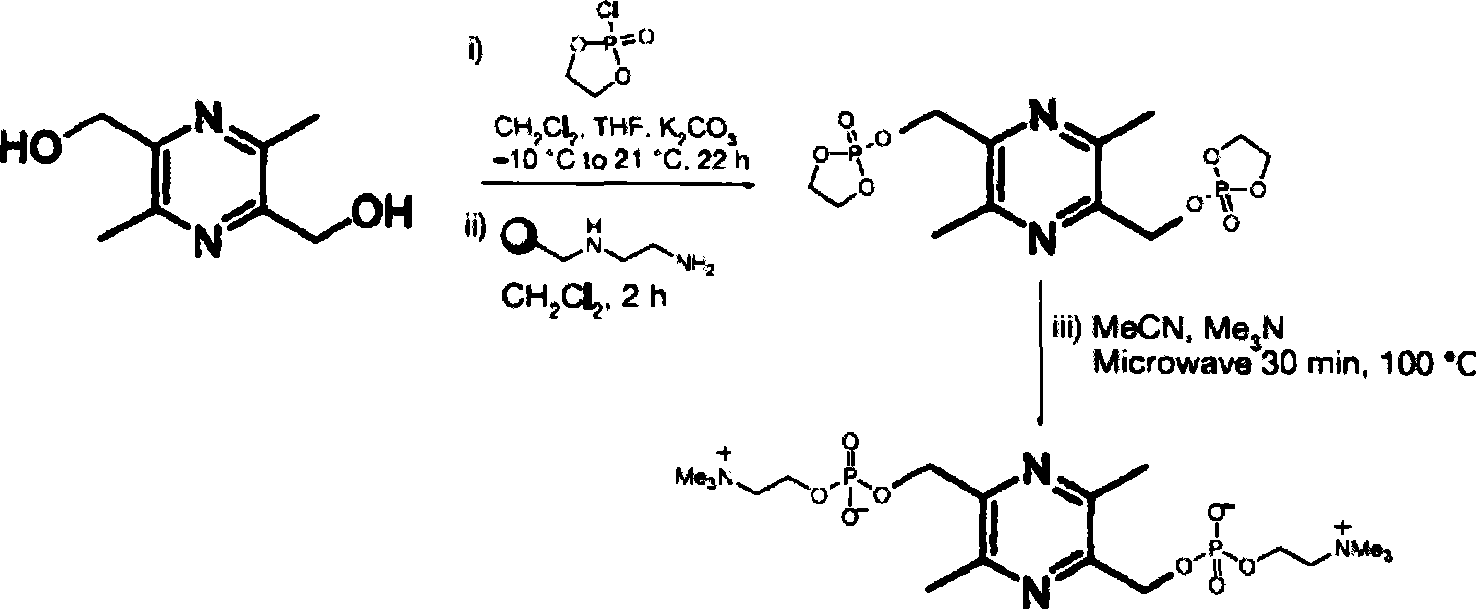
[0012] 1. Preparation of intermediates
[0013] Ethyl chlorinated phosphate was dissolved in dichloromethane at a concentration of about 21mmoL, and this solution was added to a suspension of dichloromethane containing pyridine (about 20.8mmoL) to prepare a reaction solution, which was stirred continuously. The raw material 2,5-dimethylol-3,6-dimethylpyrazine was dissolved in tetrahydrofuran (about 6.1mmoL), and then the solution was added dropwise into the reaction solution at -30°C, heated to room temperature, and stirred for 18h .
[0014] Add N-(2-aminoethyl)aminomethyl polystyrene resin and dichloromethane, shake vigorously for 2h, and use the solution Filtrate through celite, elute with diethyl ether, and concentrate the filtrate in vacuo to ointment of orange-yellow phosphorylated intermediate.
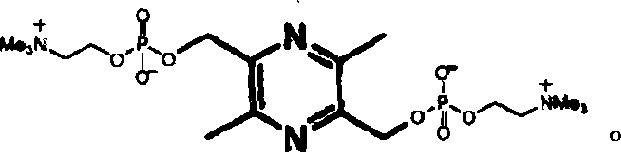
[0015] 2. Preparation of 2,5-bis(phosphorylcholine)methyl-3,6-dimethylpyrazine
[0016] Dissolve the above orange-yellow phosphorylated intermediate ointment in anhydrous a...
Embodiment 1
[0018] Ethyl phosphate chloride (2mL, 21mmoL) was mixed with dichloromethane (40mL) containing pyridine (1.08mL, 20.8mmoL) to prepare a reaction solution, which was cooled to -30°C and kept stirring. 2,5-Hydroxymethyl-3,6-dimethylpyrazine (1 g, 6.1 mmoL) was dissolved in 10 mL of tetrahydrofuran, then added dropwise to the above reaction solution, heated to room temperature, and stirred for 18 h. Then add N-(2-aminoethyl) aminomethyl polystyrene resin (4.5g) and dichloromethane (10mL), shake for 2h, and the solution Filter with 2 g of diatomaceous earth, elute with diethyl ether, and concentrate the filtrate in vacuo to obtain 0.95 mg of an orange-yellow phosphorylated intermediate ointment.
[0019] Dissolve the above orange-yellow phosphorylated intermediate ointment in anhydrous acetonitrile (10 mL), then add trimethylamine (10 mL), seal the reaction solution and heat to 100° C. for 30 min with microwave. The supernatant was poured out, and the dark yellow residue was dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com