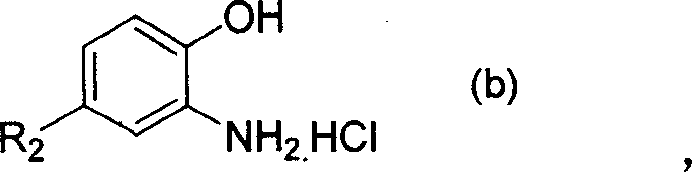Synthesis method for dibenzoxazole compounds
A synthesis method and benzoxazole technology are applied in the synthesis field of bisbenzoxazole compounds, can solve the problems of long reaction time, unfavorable environment, large amount of alkali, etc., and achieve short reaction time, less three wastes and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
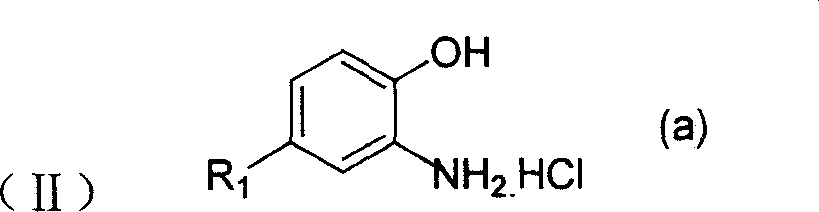
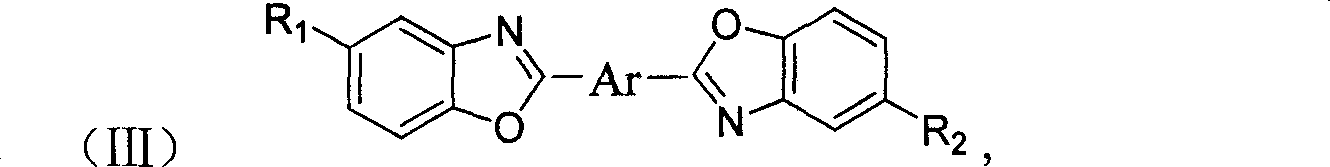
[0035] 11.5 g (0.05 mol) of 4,4'-dicyanostyrene, 16 g (0.11 mol) of o-aminophenol hydrochloride, and 90 g of nitrobenzene were respectively put into a 150 ml three-necked bottle. Sealed, vacuumed, replaced with nitrogen. Raise the temperature to 190-200°C, stir rapidly and keep warm for 3 hours, and the reaction is completed. Cool down, filter and dry. 19.5 g of 4,4'-bis(benzoxazol-2-yl)stilbene was obtained.
example 2
[0037] 9 g (0.05 mol) of naphthalene-1,4-dinitrile, 16 g (0.11 mol) of o-aminophenol hydrochloride, and 90 g of sulfolane were respectively put into a 150 ml three-necked flask. Sealed, vacuumed, replaced with nitrogen. Raise the temperature to 200-230° C., stir rapidly and keep warm for 3 hours, and the reaction is completed. 17 g of 1,4-bis(benzoxazol-2-yl)naphthalene was obtained.
example 3
[0039] In a 150ml three-necked bottle, 11.5g (0.05mol) of 4,4'-dicyano-stilbene, 8g (0.055mol) of o-aminophenol hydrochloride, and 8.8g (0.055mol) of o-amino-p-cresol hydrochloride ), sulfolane 80g. Sealed, vacuumed, replaced with nitrogen. Raise the temperature to 190-200°C, stir rapidly and keep warm for 3 hours, and the reaction is completed. Cool down, filter and dry. 19 g of a bisbenzoxazole stilbene mixture was obtained.
[0040] Determine the percentage composition of the mixture with liquid chromatography as:
[0041] 4-(Benzoxazol-2-yl)-4'-(5-methylbenzoxazol-2-yl)stilbene 49%; 4,4'-bis(benzoxazol-2- base) stilbene 24%; 4,4'-bis(5-methylbenzoxazol-2-yl) stilbene 27%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com