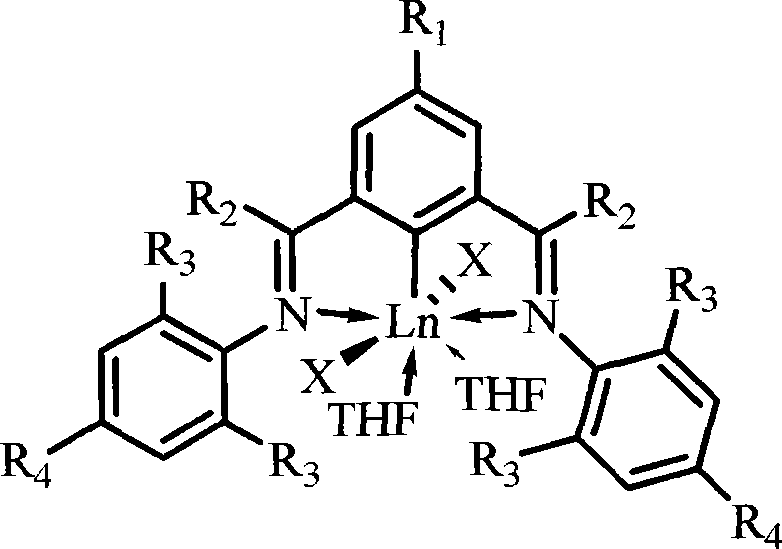
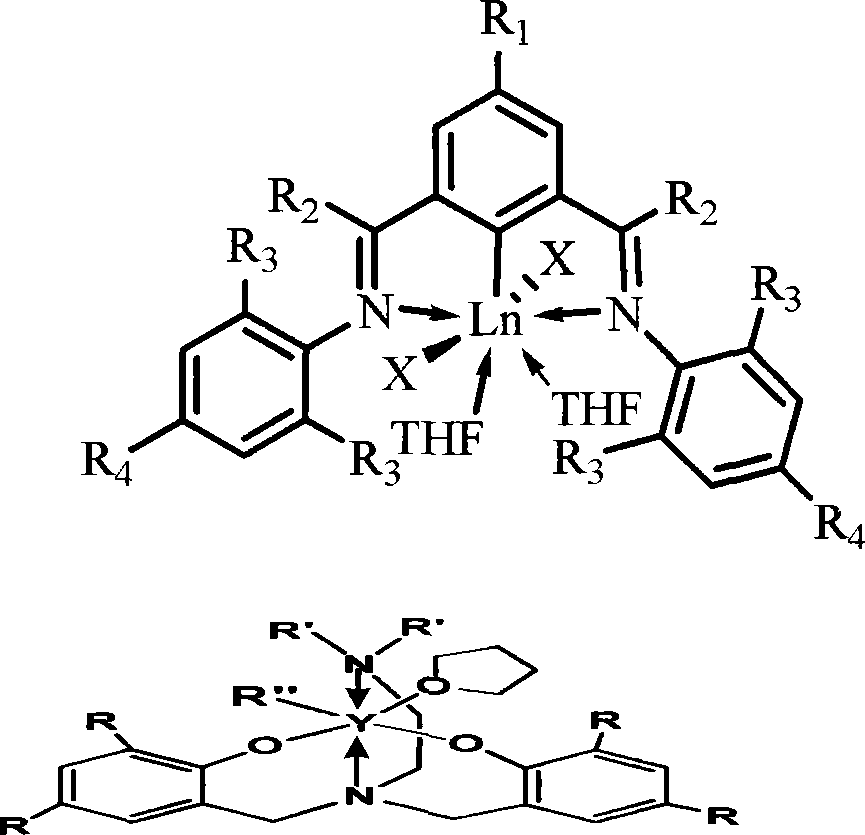
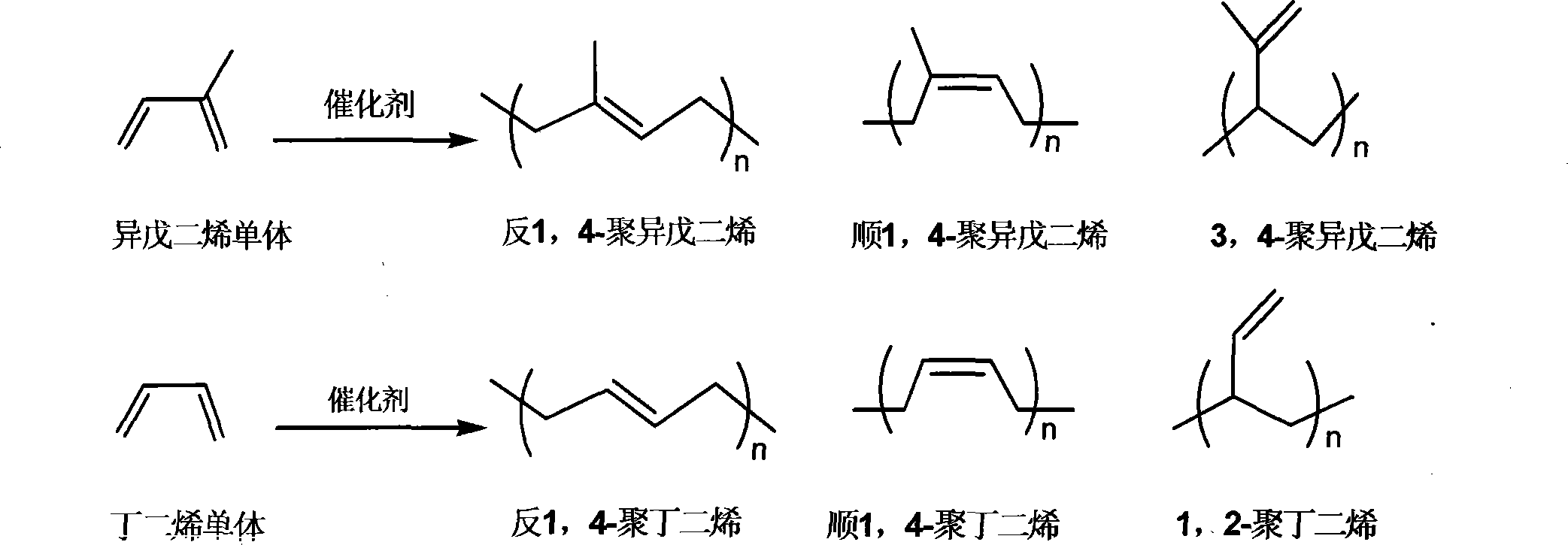
Rare earth catalyst for catalyzing syn form 1,4-selectivity polymerization of isoprene or butadiene
A technology of rare earth catalysts and isoprene, applied in the field of rare earth catalysts, can solve the problems of catalyst activity improvement and achieve the effects of reduced selectivity, high stability and high polymerization activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0041] Preparation Example 1 Preparation of Complex 1
[0042]
[0043] Preparation Example 1
[0044] At 0°C, a 1.5 mol / L hexane solution of butyl lithium (0.8 mL, 1.2 mmol) was added dropwise to the 2,6-(N-2,6-dimethyl)imino group 1 -A suspension of bromobenzene (0.5 g, 1.2 mmol) in hexane (20 mL). The reaction solution was reacted at this temperature for 4 hours and the YCl 3 (THF) 3.5 (0.64g, 1.44mmol) was added to the above reaction solution, the reaction solution naturally rose to room temperature and continued to react for 10 hours, the solvent was removed in vacuo, the residue was extracted with toluene, and the toluene solution was concentrated to obtain a total of 0.57g of bright yellow crystal complex 1 , yield 74%. Elemental analysis, its molecular formula is C 32 h 37 Cl 2 N 2 o 2 Y(%): C, 59.53; H, 5.74; N, 4.31.
preparation Embodiment 2
[0045] Preparation Example 2 Preparation of Complex 2
[0046]
[0047] At 10°C, at 0°C, a 1.5mol / L hexane solution of butyllithium (0.8mL, 1.2mmol) was added dropwise to 2,6-(N-2,6-diethyl 1-bromobenzene imino (0.57 g, 1.2 mmol) in hexane (20 mL). The reaction solution was reacted at this temperature for 6 hours and the YCl 3 (THF) 3.5 (0.64g, 1.44mmol) was added to the above reaction solution, the reaction solution naturally rose to room temperature and continued to react for 8 hours, the solvent was removed in vacuo, the residue was extracted with toluene, and the toluene solution was concentrated to obtain a total of 0.58g of bright yellow crystal complex 2 , yield 70%. Elemental analysis, its molecular formula is C 36 h 45 Cl 2 N 2 o 2 Y: C, 61.92; H, 6.47; N, 4.09.
preparation Embodiment 3
[0048] Preparation Example 3 Preparation of Complex 3
[0049]
[0050] At 0°C, a hexane solution (0.8 mL, 1.2 mmol) of butyllithium with a concentration of 1.5 mol / L was added dropwise to 2,6-(N-2,6.diisopropyl)imino 1-Bromobenzene (0.64 g, 1.2 mmol) was suspended in hexane (20 mL). The reaction solution was reacted at this temperature for 3 hours and the YCl 3 (THF) 3.5 (0.64g, 1.44mmol) was added to the above reaction solution, the reaction solution naturally rose to room temperature and continued to react for 8 hours, the solvent was removed in vacuo, the residue was extracted with toluene, and the toluene solution was concentrated to obtain a total of 0.54g of bright yellow crystal complex 3 , yield 60%. Elemental analysis, its molecular formula is C 40 h 53 Cl 2 N 2 o 2 Y: C, 63.64; H, 6.98; N, 3.63.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com