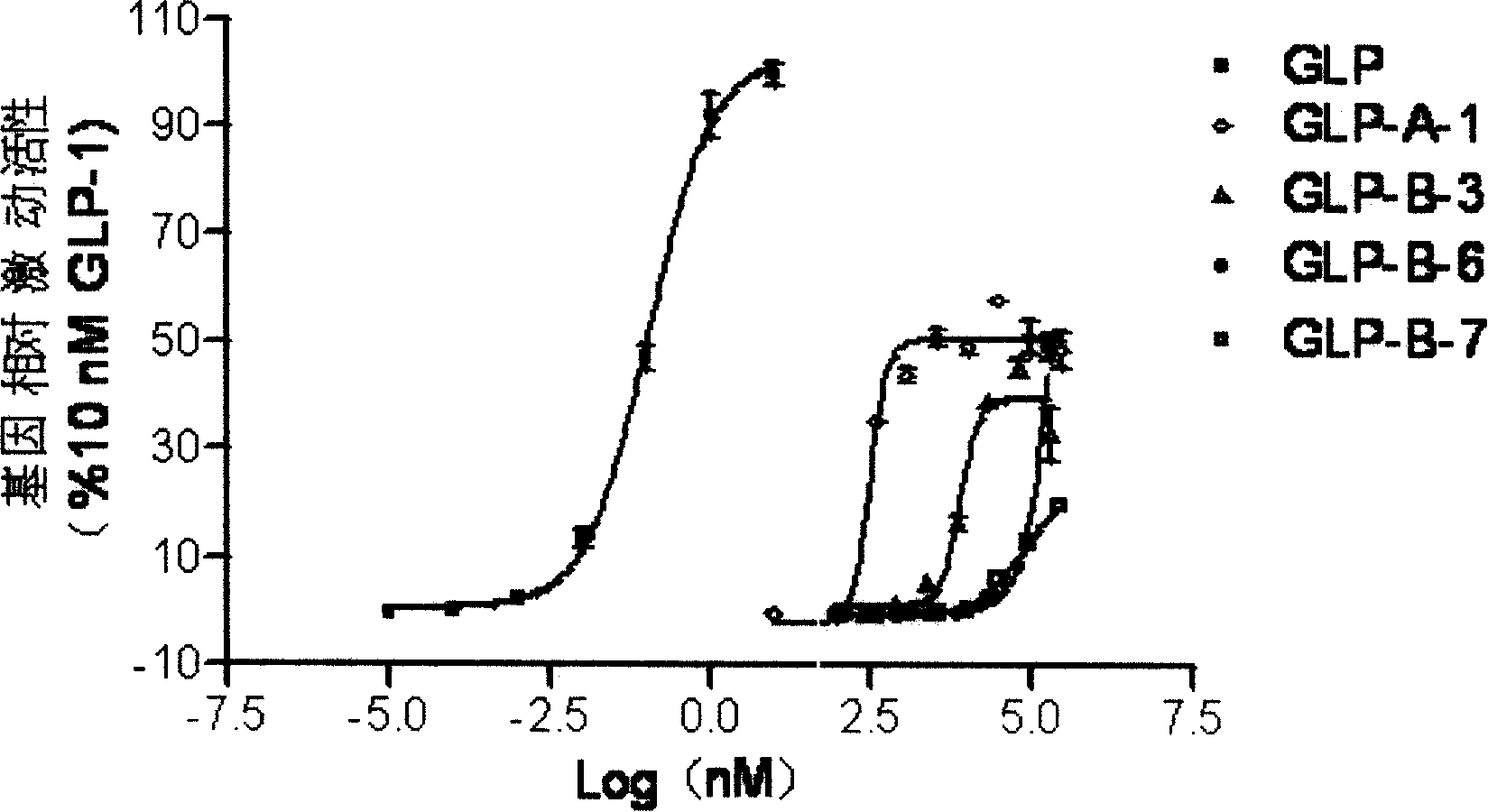
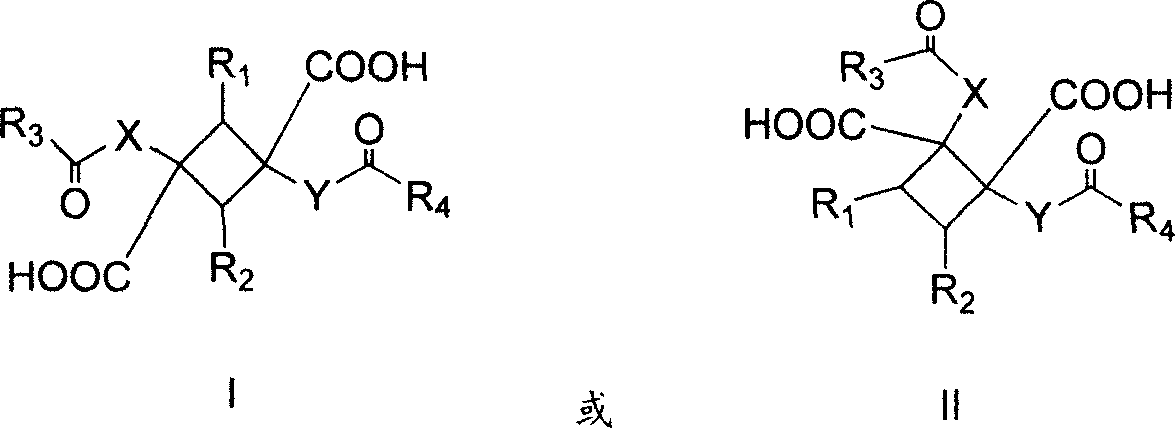
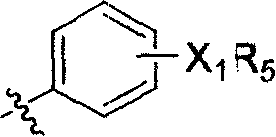Compound with substituted cyclobutane structure, production method and medicine use thereof
A kind of technology of cyclobutane and compound, applied in a kind of compound with substituted cyclobutane structure, and the field of preparation and medical use thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0163] Embodiment 1: the preparation of compound GLP-A-1
[0164] NMR calibration: δH 2.50ppm (DMSO-d 6 ).
[0165]
[0166] Compound 0601 (1 g) was dissolved in an appropriate amount of DMSO, placed under a 150W high-pressure mercury lamp for 3 days of irradiation, added 1 ml of water, and continued to irradiate for 25-30 days, during which the reaction was detected and followed by HPLC. After the reaction was completed, the solvent was removed by lyophilization, and the residue was separated by reverse phase silica gel column chromatography. Compound GLP-A-1 was obtained as light yellow powdery solid.
[0167] 1 HNMR (300MHz, DMSO-d 6 ) 10.118 (2H, br.s), 8.615 (2H, br.s), 8.095 (2H, dd, J 1 = 4.8Hz,J 2 =1.2Hz), 8.027(2H,dd,J 1 =3.9Hz,J 2= 1.5Hz), 7.569 (4H, d, J = 8.4Hz), 7.365 (4H, d, J = 8.7Hz), 7.318 (2H, dd, J 1 =3.9Hz,J 2 =5.1Hz), 7.280(2H, m), 7.260(2H, m), 7.203(2H, br.d, J=8.1Hz), 4.981(2H, s), 3.228(6H, s), 2.015(6H , s).
Embodiment 2
[0168] Embodiment 2: the preparation of compound GLP-A-2
[0169] NMR calibration: δH 2.50ppm (DMSO-d 6 ).
[0170]
[0171] Compound 0602 (10 g) was dissolved in an appropriate amount of DMSO, placed under a 150W high-pressure mercury lamp for 3 days of irradiation, added 1 ml of water, and continued to irradiate for 25-30 days, during which the reaction was detected and followed by HPLC. After the reaction was completed, the solvent was removed by lyophilization, and the residue was separated by reverse phase silica gel column chromatography. Compound GLP-A-2 was obtained as light yellow powdery solid.
[0172] 1 H NMR (300MHz, DMSO-d 6 )δ8.770 (2H, br.s), 8.090 (2H, d, J=5.1Hz), 8.026 (2H, d, J=3.9Hz), 7.2-7.5 (18H, m), 5.011 (2H, s ), 3.231 (6H, s).
Embodiment 3
[0173] Embodiment 3: the preparation of compound GLP-B-1
[0174] NMR calibration: δH 2.50ppm (DMSO-d 6 ).
[0175]
[0176] Compound 0603 (1.5g) was dissolved in an appropriate amount of DMSO, placed under a 150W high-pressure mercury lamp for 3 days of irradiation, added 1ml of water, and continued to irradiate for 25-30 days, during which the reaction was detected and followed by HPLC. After the reaction was completed, the solvent was removed by lyophilization, and the residue was separated by reverse phase silica gel column chromatography. Compound GLP-B-1 was obtained as light yellow powdery solid.
[0177] 1 H NMR (300MHz, DMSO-d 6 )δ9.532 (2H, br.s), 8.433 (2H, br.s), 7.534 (4H, m), 7.428 (4H, d, J=8.7Hz), 7.329 (4H, d, J=8.4Hz ), 7.234 (6H, m), 4.912 (2H, s), 1.453 (18H, s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com