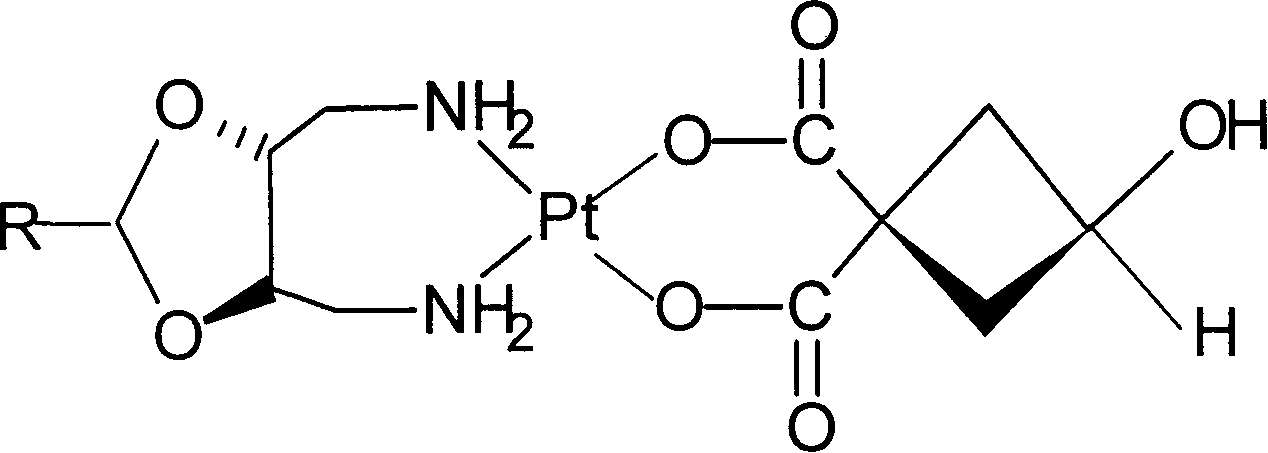
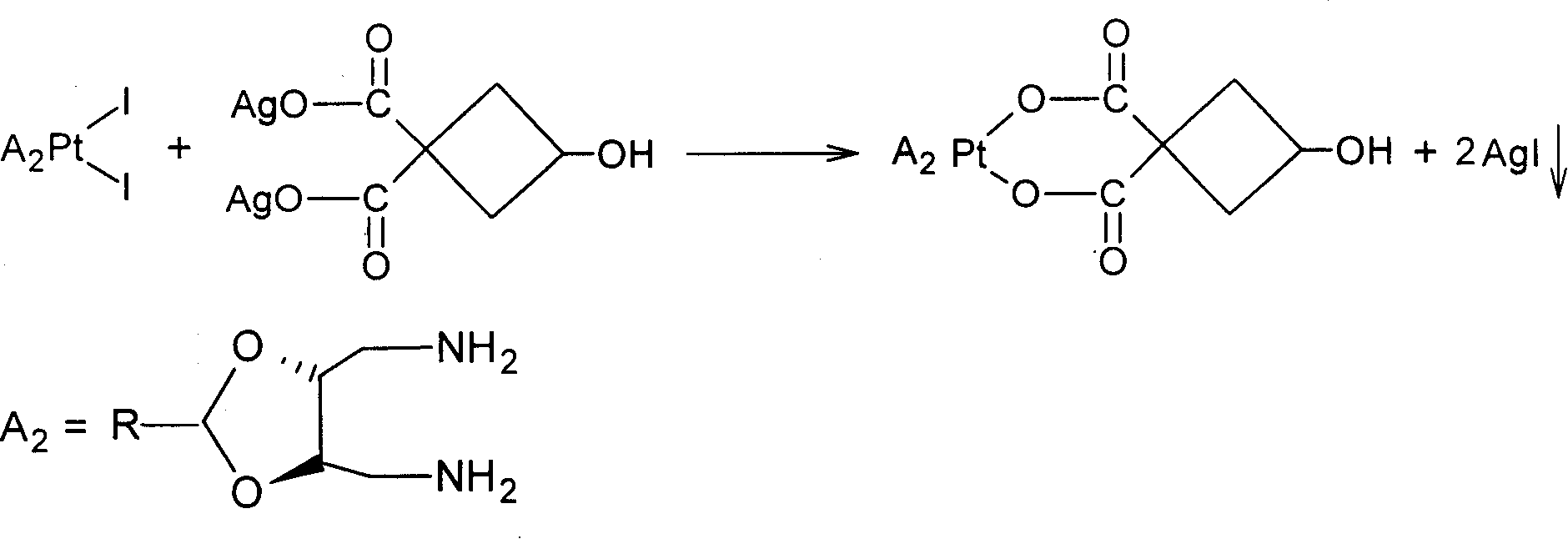
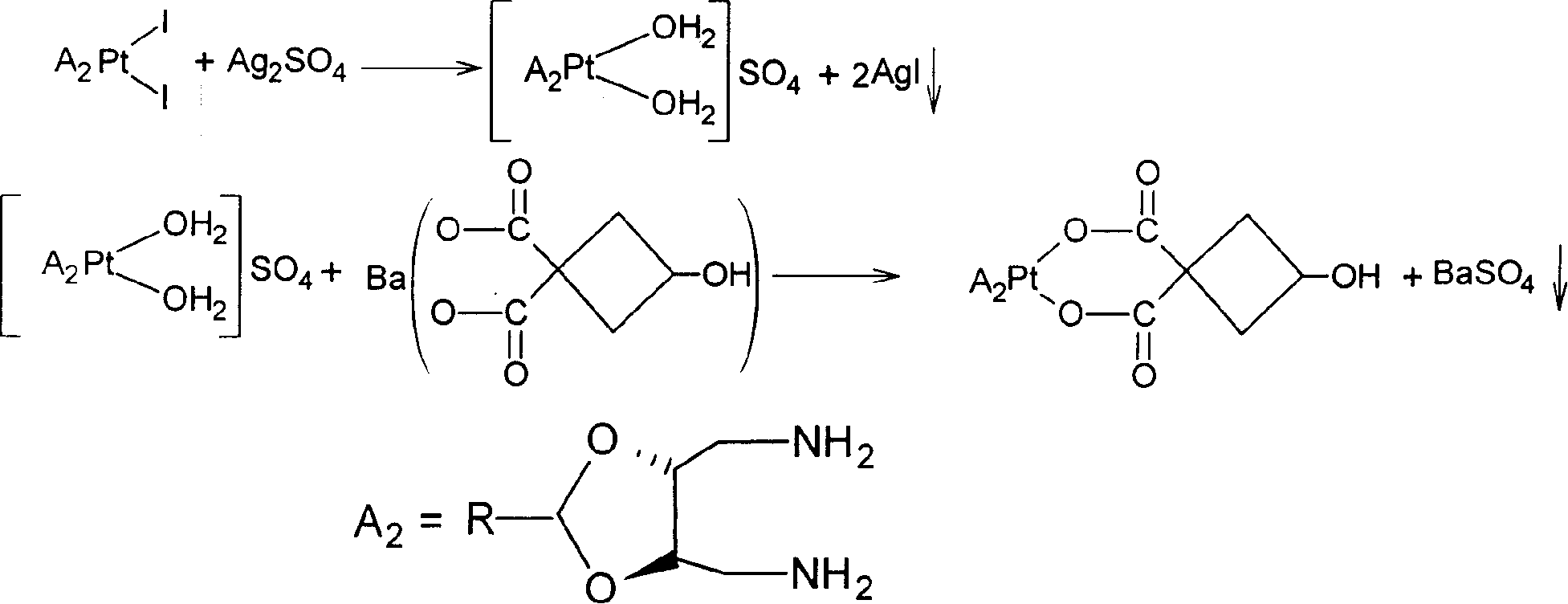
Novel water soluble Pt (II) anti-cancer ligand and its preparing method
A technology of complexes and substituents, which is applied in the field of platinum anticancer complexes and their preparation, can solve the problems of high toxicity and side effects, and the overall curative effect is not as good as that of carboplatin, and achieve the effects of simple operation, high yield and pure product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] (1) Preparation of 3-hydroxyl-1,1-silver cyclobutane dicarboxylate
[0019] Firstly, 3-hydroxy-1,1-cyclobutanedicarboxylic acid (mp 151°C-152°C) was prepared according to the method reported in the literature [Inorganica Chimica Acta, 2004, 357, 4452-4466]. Take 10g of 3-hydroxy-1,1-cyclobutanedicarboxylic acid, dissolve it in 100ml of water, adjust the pH to 6-7 with 1mol / L NaOH, add 130mmol, 100ml of AgNO 3 (excessive 5%), 3-hydroxy-1,1-cyclobutane dicarboxylate silver precipitate was obtained, collected by filtration, washed with water and ethanol, and dried under vacuum at 60-70°C for 4 hours to obtain 21.7g 3-hydroxy- Silver 1,1-cyclobutanedicarboxylate, yield 94.2%.
[0020] (2)cis-[Pt(II)A 2 I 2 ] Preparation of intermediate
[0021] [J.Med.Chem., 1994,37,1471-1485] synthetic carrier group A according to the process route reported in the literature 2 [A 2 =2-methyl-(4R,5R)-4,5-bis(aminomethyl)-1,3-dioxolane; 2-ethyl-(4R,5R)-4,5-bis( aminomethyl)-1,3-dioxol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com