Process of stereospecific synthesis of sapogenins
A kind of technology of aglycone and saponin, applied in the field of stereospecific synthesis of saponin, can solve problems such as difficult separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
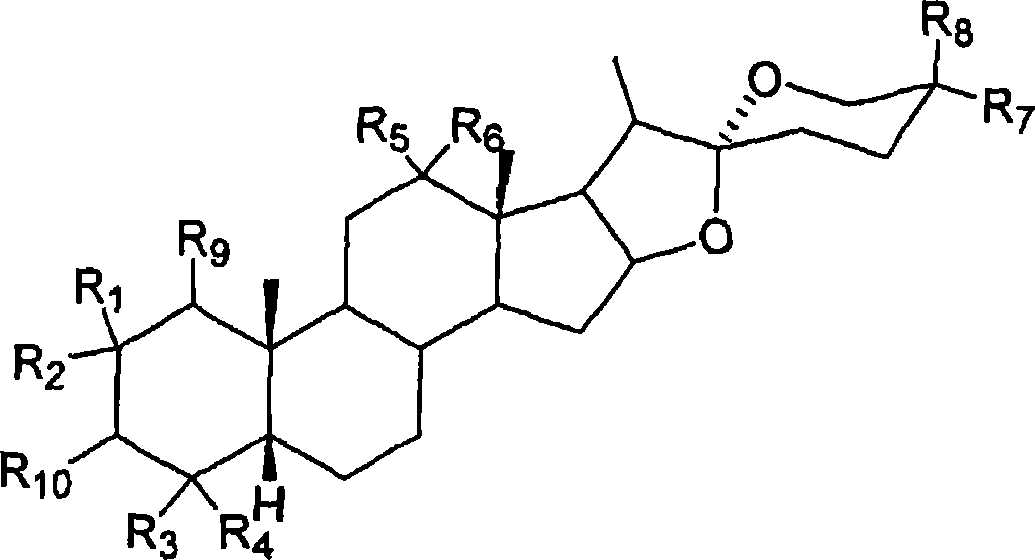
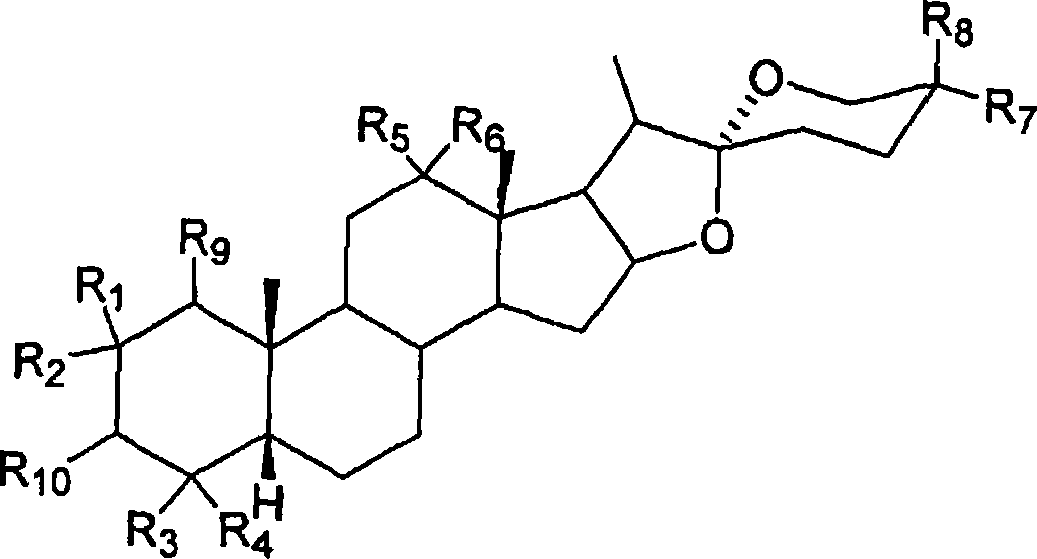
[0049] In the reaction step of preparing the required saponin in the first aspect of the present invention, as the starting material of this step, 3-ketone, 5β-H steroidal saponin is preferably except for 3- All positions other than the position group are consistent with all positions of the required saponin. However, if necessary or desired, appropriate protecting groups can be used for reduction and then removed to obtain the desired saponin.
[0050] The term "protecting group" as used herein refers to a group used to protect reactive functional groups such as hydroxyl or carboxyl groups to avoid unnecessary participation in the reaction when they are desired groups in the final product. Conventional protecting groups can be used according to standard practice, and specific examples can be found in T.W. Green and P.G.M. Wuts in "Protective Groups in Organic Chemistry" John Wiley and Sons, 1991; J.F.W McOmie in "Protective Groups in Organic Chemistry" Plenum Press, 1973.
...
Embodiment 1
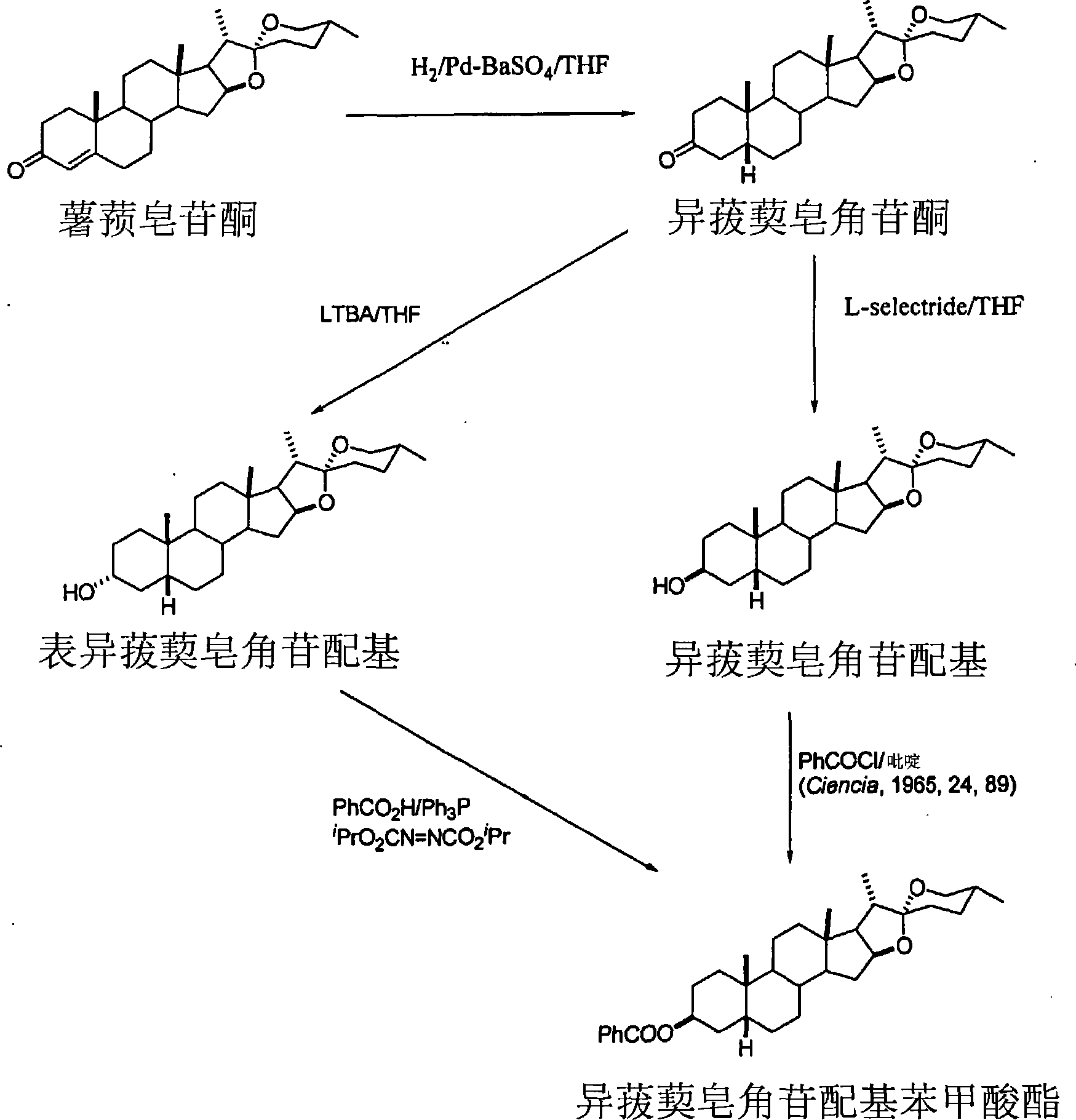
[0088] Synthesis of smilagenin from smilagenone with L-Selectride® at -10°C
[0089] Smilax saponinone (657 g) was dissolved in tetrahydrofuran (4000 ml), the solution was purged with nitrogen, and the solution was cooled until the internal temperature reached about -10°C. To the solution was added L-Selectride(R) (1 M in THF, 2400 mL) over about 50 minutes and stirred for 90 minutes. A solution of citric acid (600 g) in water (2000 ml) was added slowly, keeping the temperature below 0°C. The mixture was warmed to room temperature and stirred for 30 minutes. The aqueous layer was separated and extracted with dichloromethane (2000ml) and the layers were separated. The aqueous layer was extracted with dichloromethane (1500ml). The combined organic extracts were washed with water (4000ml) and washed with MgSO 4 dry. Evaporate the organic extract to obtain the different smilax saponin.
Embodiment 2
[0091] Synthesis of smilagenin from smilagenone with K-Selectride® at -15°C
[0092] To a solution of smilaxaponinone (500 g) in tetrahydrofuran (3500 ml) was added K-Selectride(R) (1600 ml, 1M in THF) under a nitrogen atmosphere at a temperature of about -15°C. The reaction mixture was stirred at this temperature for 30 minutes. Quench with aqueous citric acid (393 g citric acid dissolved in 1300 ml water), keeping the internal temperature around 0°C. The mixture was warmed to room temperature and the THF was evaporated at atmospheric pressure until a solid precipitated. The solid was filtered and pumped dry.
[0093] The solid was dissolved in dichloromethane (DCM) (6000ml), dried (MgSO 4 ) and evaporated to give a white solid which was recrystallized in isopropanol (IPA) (5000ml) to give smilaxa saponin.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com