Cell vaccine for preventing and controlling hepar damnification
A technology of liver injury and cell population, which is applied in the fields of biotechnology and medicine, and can solve problems such as the preparation of vaccines in hepatitis models
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] The preparation of embodiment 1 lymphocyte vaccine
[0083] Prepare CIH model:
[0084] Concanavalin A (15 mg / kg body weight, purchased from Sigma Company) or PBS was injected into the tail vein of C57BL / 6 mice (purchased from Shanghai Slack Experimental Animal Co., Ltd.) to induce CIH model.
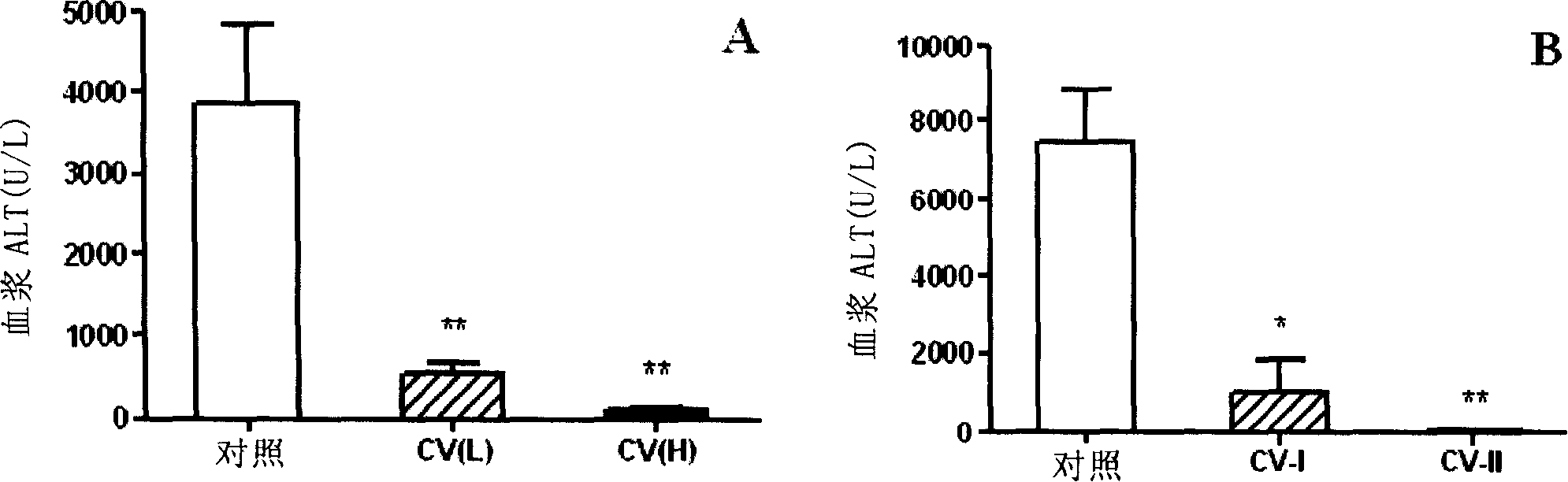
[0085] Preparation of Vaccine 1:
[0086] After inducing the CIH model for 17 hours, the spleen of the mouse was taken out, placed in PBS, and the spleen was ground through a nylon mesh to obtain a cell suspension, and red blood cell lysate was added to remove red blood cells to obtain a mononuclear cell suspension, and the concentration of the cells was adjusted. 5×10 6 / 200μl and 1×10 7 / 200μl, irradiated 4000rad as spleen-derived lymphocyte vaccine (CV).
[0087] Preparation of Vaccine 2:
[0088] 17 hours after the induction of the CIH model, before taking out the mouse spleen, the peripheral blood of the mouse was obtained by collecting blood from the heart, anticoagula...
Embodiment 2
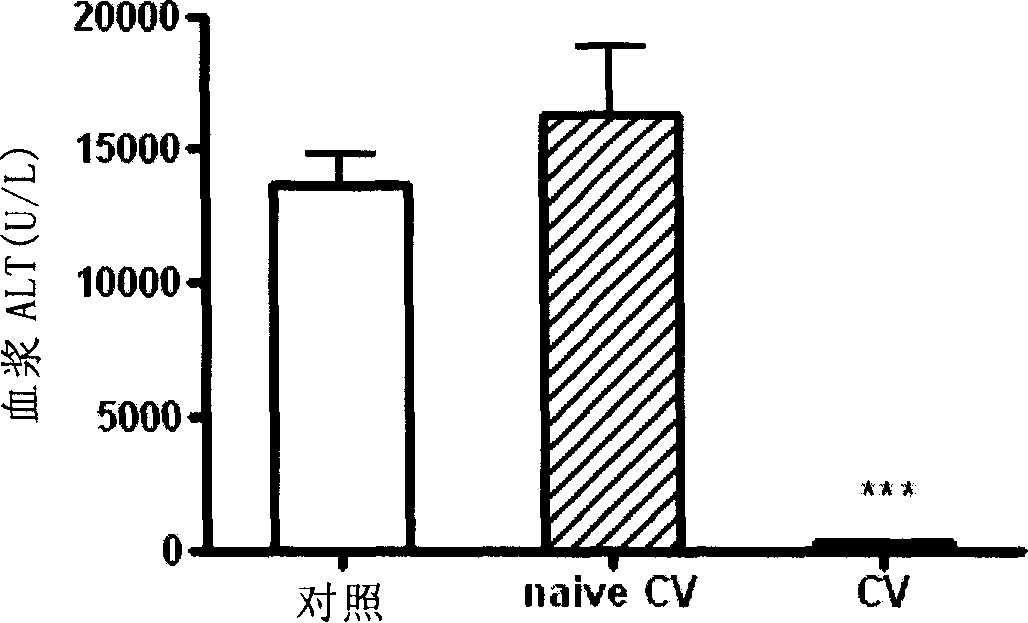
[0089]Example 2 Cell vaccine can reduce the level of alanine aminotransferase (ALT) in plasma
[0090] The cellular vaccine was prepared according to the method described in Example 1, and the C57BL / 6 mice were divided into three groups, four in each group:
[0091] Control group: inject PBS;
[0092] Pathogenic lymphocyte treatment group: injection of CV;
[0093] Non-sensitized lymphocyte treatment group: inject the lymphocytes isolated from the spleen of mice that did not induce CIH ( CV).
[0094] Mice received PBS, PBS, and CV, and CV. That is: the mice in the control group only received the injection of PBS; Mice in the CV group received irradiated spleen lymphocytes (1×10 7 cells / mouse); mice in the CV group received irradiated spleen lymphocytes (1×10 7 cells / mouse).
[0095] On day 14, all mice received a tail vein injection of concanavalin A at 15 mg / kg body weight. After 17-20 hours, the plasma of the mouse was taken out to measure the level of ALT (the ...
Embodiment 3
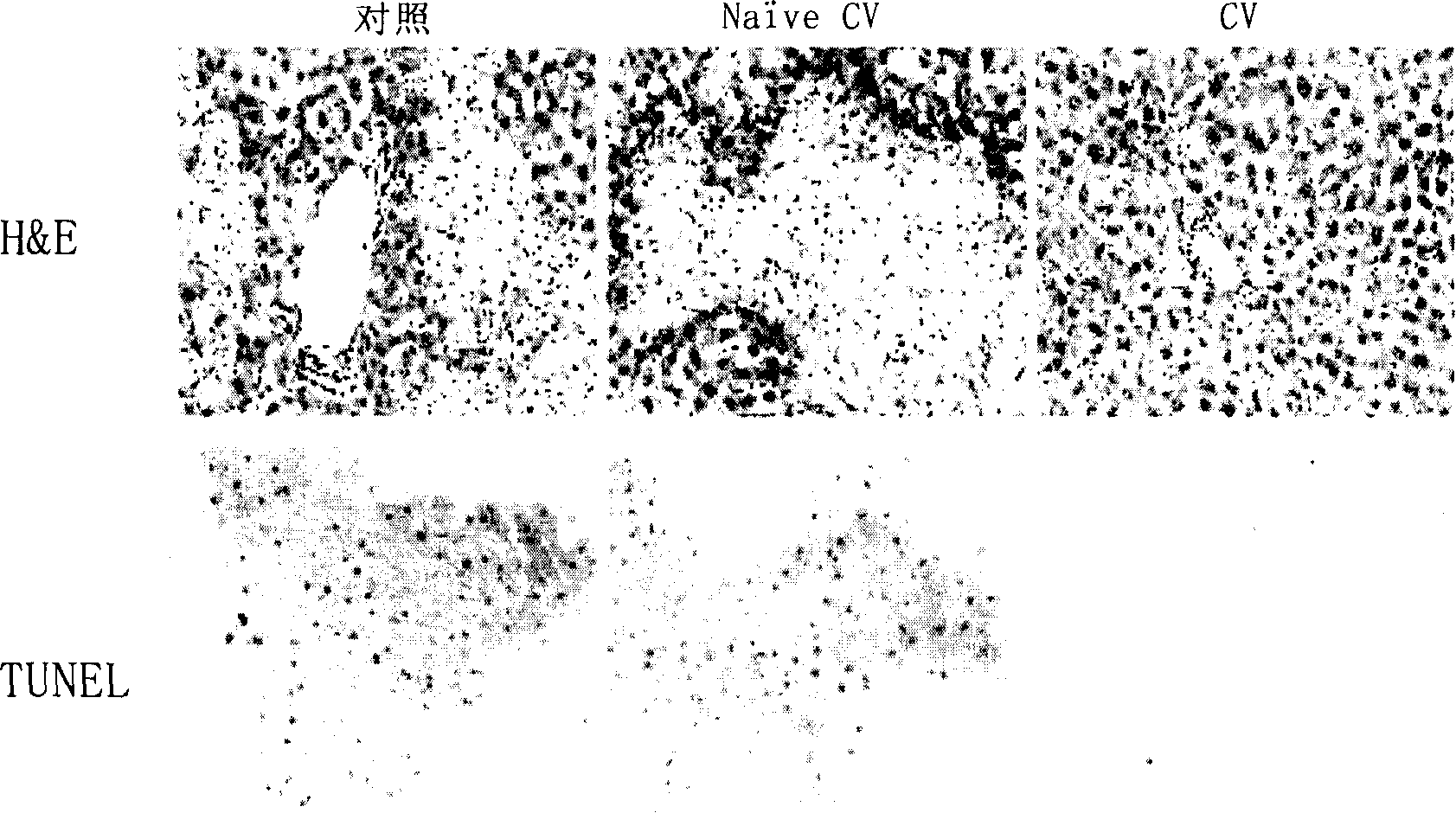
[0097] Example 3 Cellular Vaccine Can Reduce Liver Damage
[0098] The cellular vaccine was prepared according to the method described in Example 1, and the C57BL / 6 mice were divided into three groups, four in each group:
[0099] Control group: inject PBS;
[0100] Pathogenic lymphocyte treatment group: injection of CV;
[0101] Non-sensitized lymphocyte treatment group: injection cv.
[0102] Mice received PBS, PBS, and CV, and CV. That is: the mice in the control group only received the injection of PBS; Mice in the CV group received irradiated spleen lymphocytes (1×10 7 cells / mouse); mice in the CV group received irradiated spleen lymphocytes from CIH mice.
[0103] On day 14, all mice were given a tail vein injection of concanavalin A at 15 mg / kg. After 17-20 hours, the livers of the mice were removed and fixed with 4% paraformaldehyde. Tissue sections of the liver were subjected to routine H&E staining and TUNNEL staining, and were examined by light microscop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com