Function of tetrandrine in preparing liver scathing-prevention medicament
A technology of tetrandrine and liver injury, applied in the field of pharmacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] Preparation method of tetrandrine and pharmaceutically acceptable salt thereof
[0063] The preparation method of tetrandrine can adopt methods known to those skilled in the art, such as chemical synthesis or extraction from plants. Preferably, a method of extracting tetrandrine from plants can be used.
[0064] The main advantages of the present invention are:
[0065] (1) The new application of tetrandrine in the prevention and treatment of hepatitis or liver injury has been discovered for the first time. It has strong pharmacological effects and no toxic and side effects, and has excellent medicinal prospects.
[0066] (2) The tetrandrine can be directly extracted from plants, and has the advantages of low toxicity, convenient administration, abundant sources, low price and simple preparation process.
Embodiment 1
[0069] Example 1 Preparation and injection of tetrandrine
[0070] 1. Preparation of tetrandrine injection
[0071] Prepare tetrandrine injection, the method is as follows: take 100 mg of tetrandrine (purity above 98%) solid powder purchased from Shaanxi Huike Plant Development Co., Ltd., add 0.1M hydrochloric acid to dissolve, then add the solution formed after dissolution to Add it to PBS to a final concentration of 20mg / ml, and prepare tetrandrine injections.
[0072] 2. Experimental Grouping and Injection
[0073] C57b1 / 6 mice (purchased from Shanghai Slack Experimental Animal Co., Ltd.) were selected as the test objects, and the mice were randomly divided into groups of 3-5 mice, and the control group and tetrandrine treatment group 1 and Han Tetrandrine treatment group 2.
[0074] Among them, the control group injected mice intraperitoneally with PBS, the tetradrine treatment group 1 injected mice intraperitoneally with 25 mg / kg body weight of tetrandrine, and the tet...
Embodiment 2
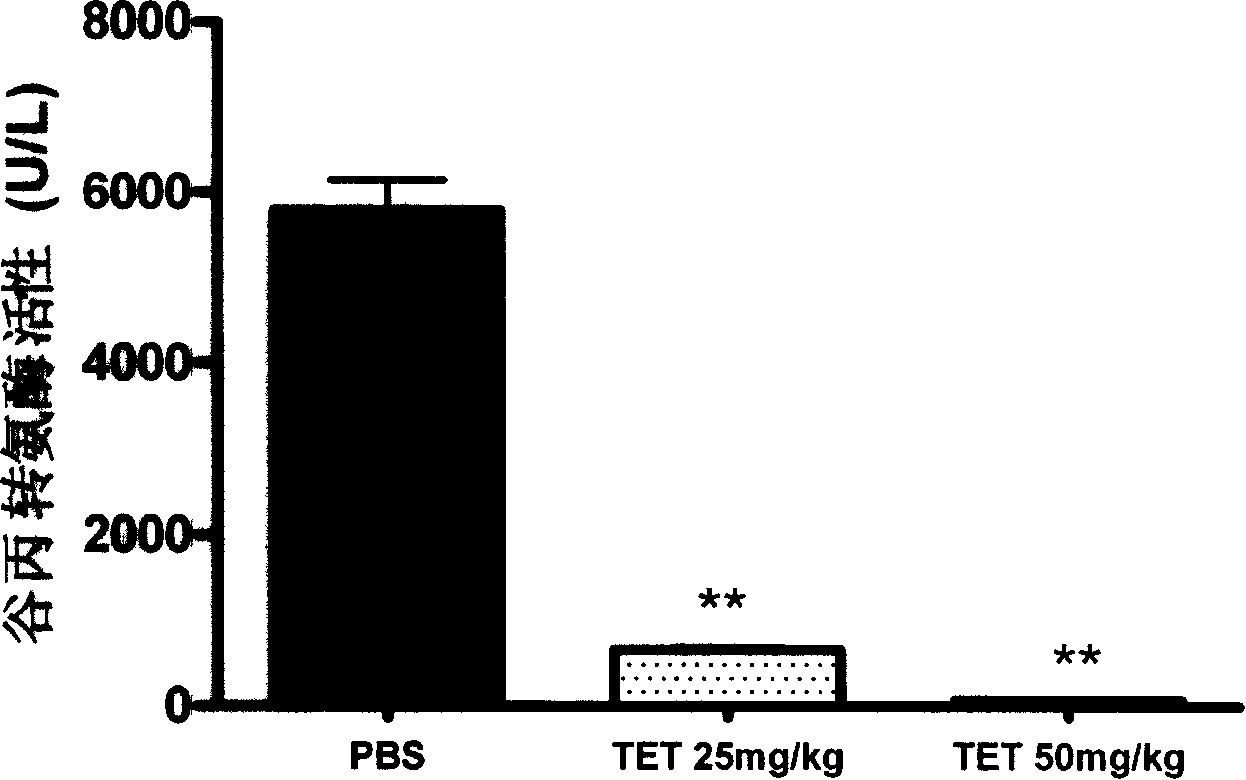
[0077] The effect of embodiment 2 tetrandrine on the activity of alanine aminotransferase (ALT) in CIH mice
[0078] In this example, the mice were treated as described in Example 1. After all the mice were injected with ConA at a dose of 15 mg / kg through the tail vein for 17 hours, the mouse serum was taken to measure the activity of alanine aminotransferase. The serum alanine aminotransferase activity assay kit produced by Hua Medical Technology Co., Ltd. was operated according to the instructions. The brief steps are as follows: After the serum is properly diluted, take 25 microliters and add it to a 96-well plate, add 100 microliters of enzyme working solution, incubate at 37 degrees Celsius for 15 minutes, add 150 microliters of stop solution, and read at 492nm on a microplate reader, according to the kit provided Calculate the alanine aminotransferase activity value using the standard serum and the dilution factor.
[0079] The data are expressed in the form of mean ± s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com