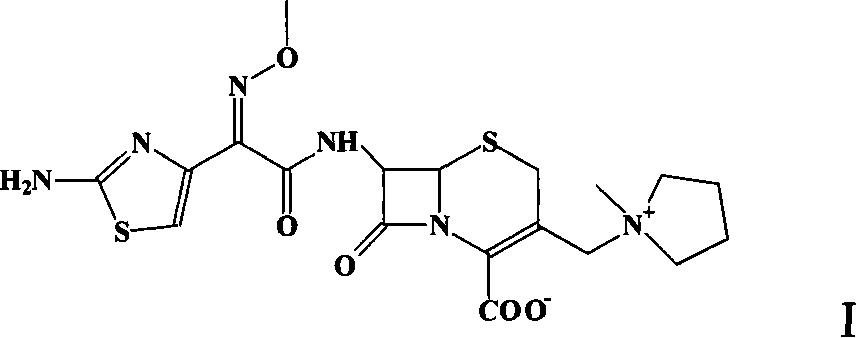
Method for preparing cefepime dihydrochloride monohydrate crystal
A technology for cefepime dihydrochloride and monohydrate, which is applied in the field of controlling the preparation of cefepime hydrochloride to generate specific hydrate crystals, and can solve the problems of reducing water content, failing to meet Chinese quality standards, and unable to guarantee crystals, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, the refining of cefepime dihydrochloride monohydrate
[0031]Add 150 g of cefepime dihydrochloride monohydrate (HPLC purity 98%, prepared according to the method in US 4910301) to 750 mL of methanol under stirring at room temperature, add an appropriate amount of activated carbon after 10 minutes, and stir for 20 minutes. Filter and wash the carbon residue with an appropriate amount of acetone-methanol 1:1 (v / v) mixed solution. Combine the filtrates and cool to 10-20°C. Add 6500 mL of acetone to the filtrate under stirring, and complete the addition within about 1 hour. Stirring was continued for 1 hour. Suction. The filter cake was washed with acetone and dried under vacuum at 40° C. to obtain about 140 g of white crystals of cefepime dihydrochloride monohydrate. HPLC purity 99.5%, water content 3.3% (K-F method), residual methanol 0.20%, residual acetone 0.35%.
Embodiment 2
[0032] Embodiment 2, prepare cefepime dihydrochloride monohydrate by cefepime monohydrochloride
[0033] 136 g of cefepime monohydrochloride (HPLC purity 97%, prepared according to the method in US 4910301) was added to 750 mL of methanol under stirring at room temperature, and 50 mL of 20% HCl was added after 10 minutes. Stir for 10 minutes, if there is any insoluble matter, filter it off. Then add 6500 mL of acetone to the solution, and add it within about 1 hour. Stirring was continued for 1 hour. Suction. The filter cake was washed with acetone and dried under vacuum at 40° C. to obtain about 130 g of white crystals of cefepime dihydrochloride monohydrate with an HPLC purity of 99%.
Embodiment 3
[0034] Embodiment 3, prepare cefepime dihydrochloride monohydrate by cefepime dihydrochloride dihydrate
[0035] Add 150g of cefepime dihydrochloride dihydrate (HPLC purity 98%, prepared by the method in US 5391729) under stirring at room temperature to the mixed solution of 150mL water and 900mL methanol, add an appropriate amount of activated carbon after 10 minutes, and stir for 30 minute. Filter and wash the carbon residue with an appropriate amount of acetone-methanol 1:1 (v / v) mixed solution. Combine the filtrates and cool to 10-20°C. Add 8500 mL of acetone to the filtrate under stirring, and complete the addition within about 1 hour. Stirring was continued for 1 hour. Suction. The filter cake was washed with acetone and dried under vacuum at 40° C. to obtain about 130 g of white crystals of cefepime dihydrochloride monohydrate with an HPLC purity of 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com