Bioenhanced compositions
A technology of composition and enhancer, which is applied in the field of bioenhancement composition, can solve problems such as unacceptable, difficult weight of oral bioavailability, increased solubility, etc., to reduce the degree of absorption, improve bioavailability, and reduce variability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1 - Solubilization of Valsartan
[0075] Solid dispersions of valsartan containing different solubility enhancers in different proportions were prepared by adding valsartan to the molten mass with continuous mixing to obtain a homogeneous dispersion. The solubility of the resulting solid dispersion was determined in 0.1N HCl.
[0076] Table 1: Solubility of valsartan with different solubility enhancers in 0.1N HCl
[0077] Solid dispersion
solubility enhancer
HLB
Solubility mcg / ml
84.60
Valsartan: Stearoyl Macrogol Glyceride USP (Gelucire 50 / 13) 1:0.5
13
73.51 *
Valsartan: Vitamin E T.P.G.S.USP / NF1: 0.5
15
230.40
Valsartan: Vitamin E T.P.G.S.USP / NF1:1
15
337.10
Valsartan: stearoyl macrogol glyceride, USP (Gelucire 50 / 13) 1:1
13
167.19
Valsartan: Polyoxyl (polyoxyl) 40 hydrogenated castor oil, USP (Cremophor
RH40) 1:1
13
181.24
Valsa...
Embodiment 2
[0082] Example 2 - Solubilization of Valsartan Using Surfactant Composition
[0083] Valsartan solid dispersions with different solubility enhancer compositions were prepared by adding valsartan to the fusion mass of the surfactant composition under continuous mixing to obtain a homogeneous dispersion. The solubility of the resulting solid dispersion was determined in 0.1N HCl.
[0084] Table 2: Solubility of valsartan in 0.1N HCl with different solubility enhancer compositions
[0085] Solid dispersion
HLB of Solubility Enhancer Composition
Solubility mcg / ml
Valsartan
84.60
Valsartan: stearyl macrogol
Oily esters, USP (Gelucire 50 /
13): SLS * , USP1:0.5:0.1
17.5
140
Valsartan: stearyl macrogol
Oily esters, USP (Gelucire 50 /
13): SLS, US P1: 1: 0.1
15.5
171.19
Valsartan: Polyoxyethylene 40 hydrogenated
Castor Oil USP (Cremophor
RH40): SLS, USP1: 0.5: 0.1
17.5
123.7
Vals...
Embodiment 3
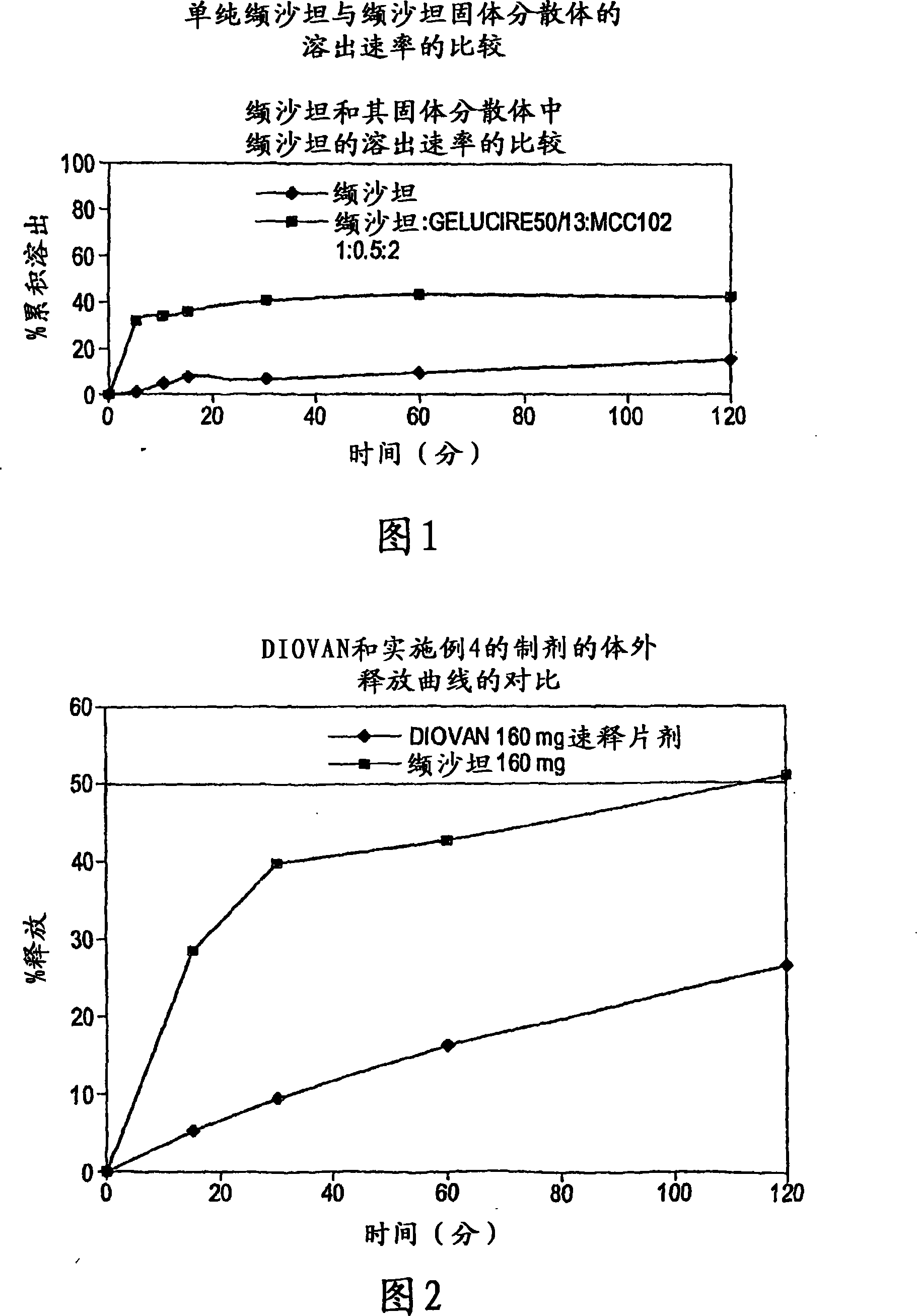
[0088] The preparation of embodiment 3-valsartan solid dispersion and its dissolution rate research
[0089] Gelucire was melted in a beaker at about 50°C on a hot plate with a thermostat and added to the melted amount of valsartan in a ratio of 1:0.5 (valsartan:Gelucire) and mixed for a while. To this mixture was added 2 parts of microcrystalline cellulose and stirred until it reached room temperature. A weighed amount of disintegrant (here 280 mg) was directly added into the dissolution tank for dissolution.
[0090] In Vitro Dissolution Studies
[0091] In vitro dissolution studies were performed according to the following instructions:
[0092] Dissolution medium: 0.1N HCl with 0.5% SLS
[0093] Dissolution Apparatus: USP Type II
[0094] Temperature: 37.5±0.5℃
[0095] RPM: 50
[0096] Sampling intervals: 5, 10, 15, 30, 60 and 120 minutes
[0097] Sampling volume: 10ml
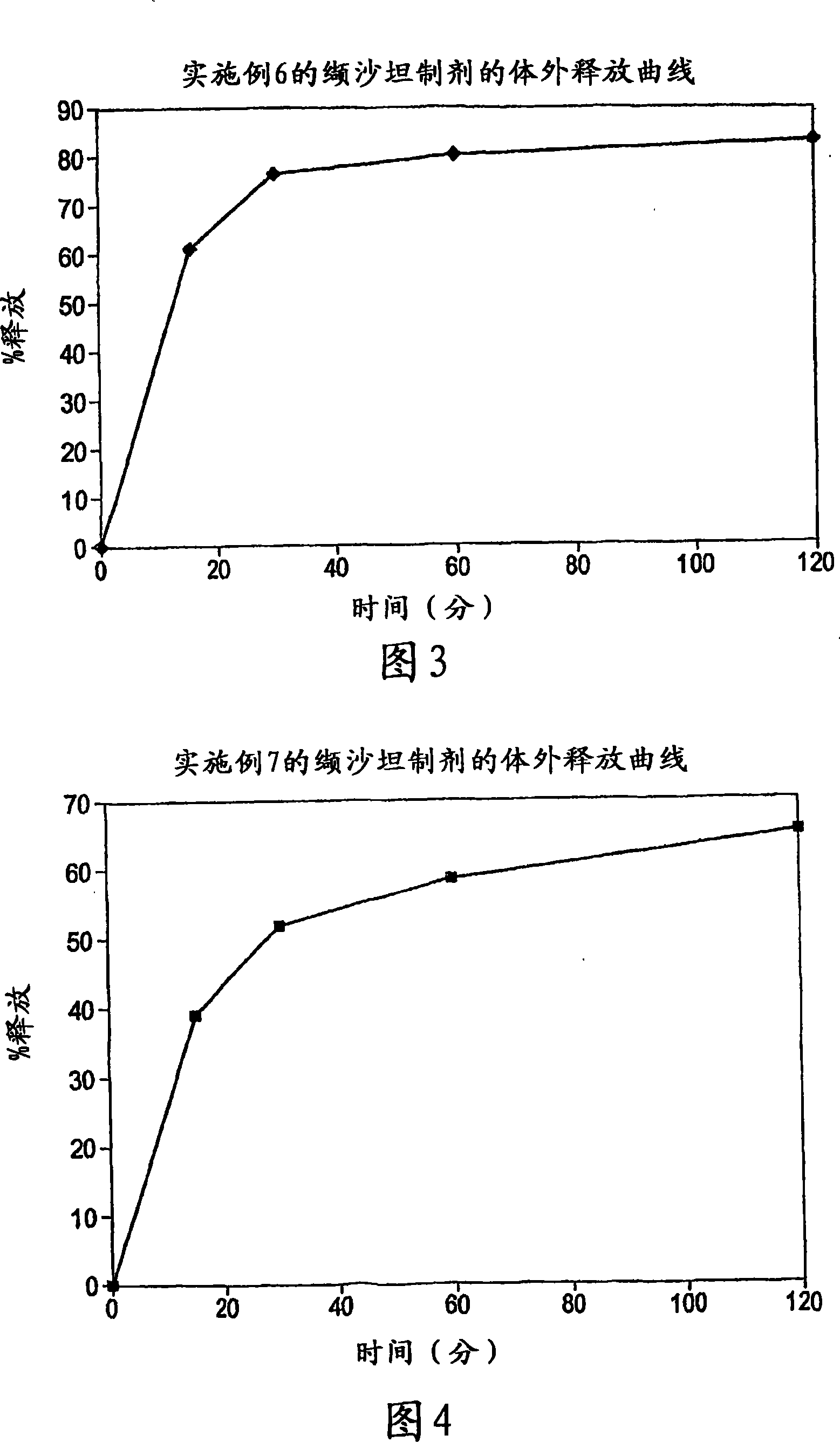
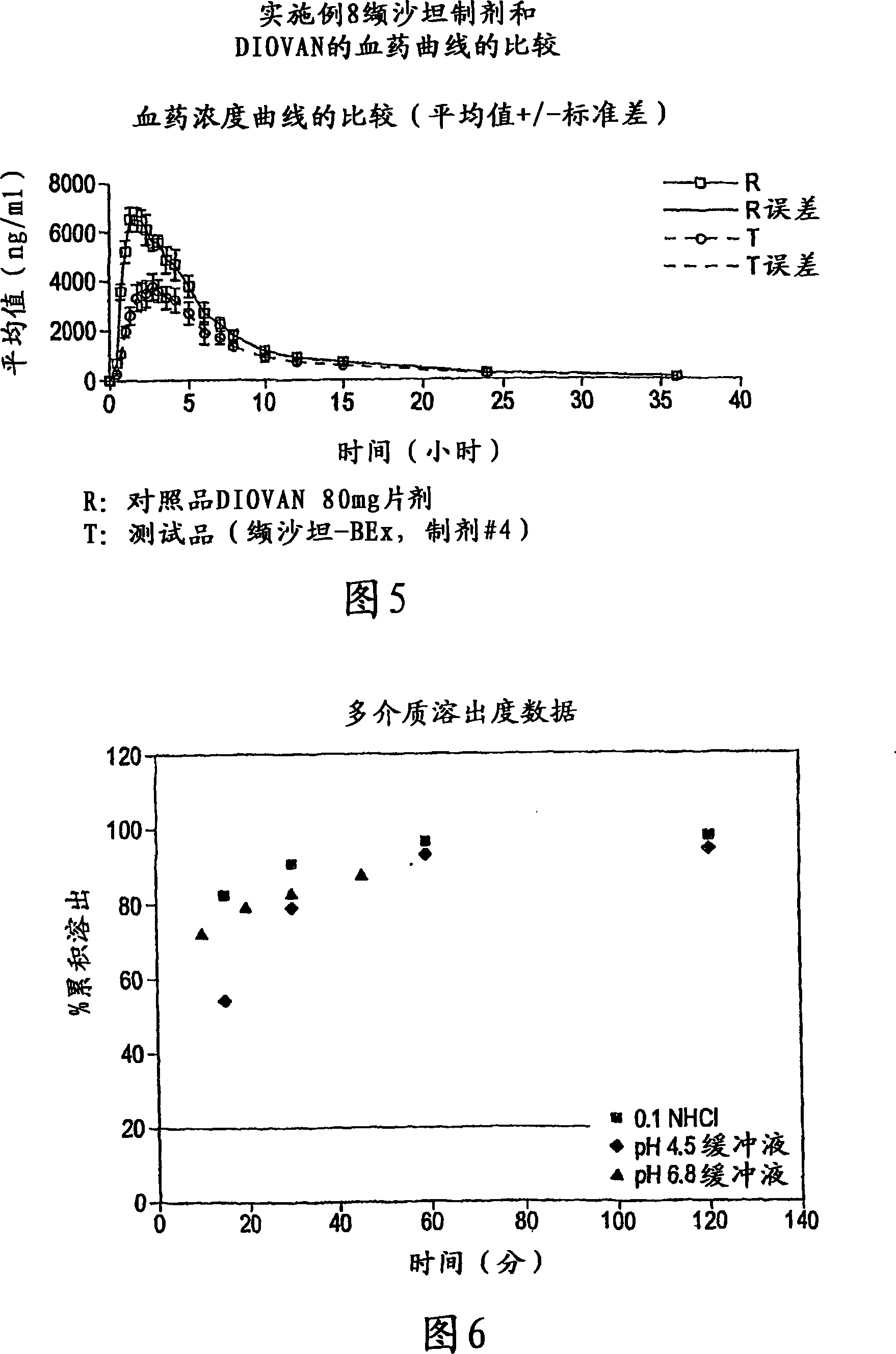
[0098] Table 3: In vitro dissolution studies of valsartan alone and valsartan solid dispersio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com